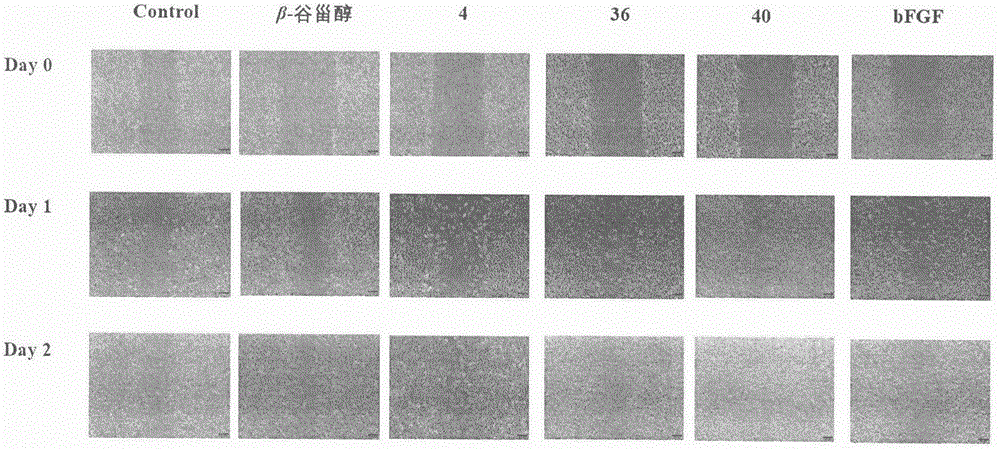
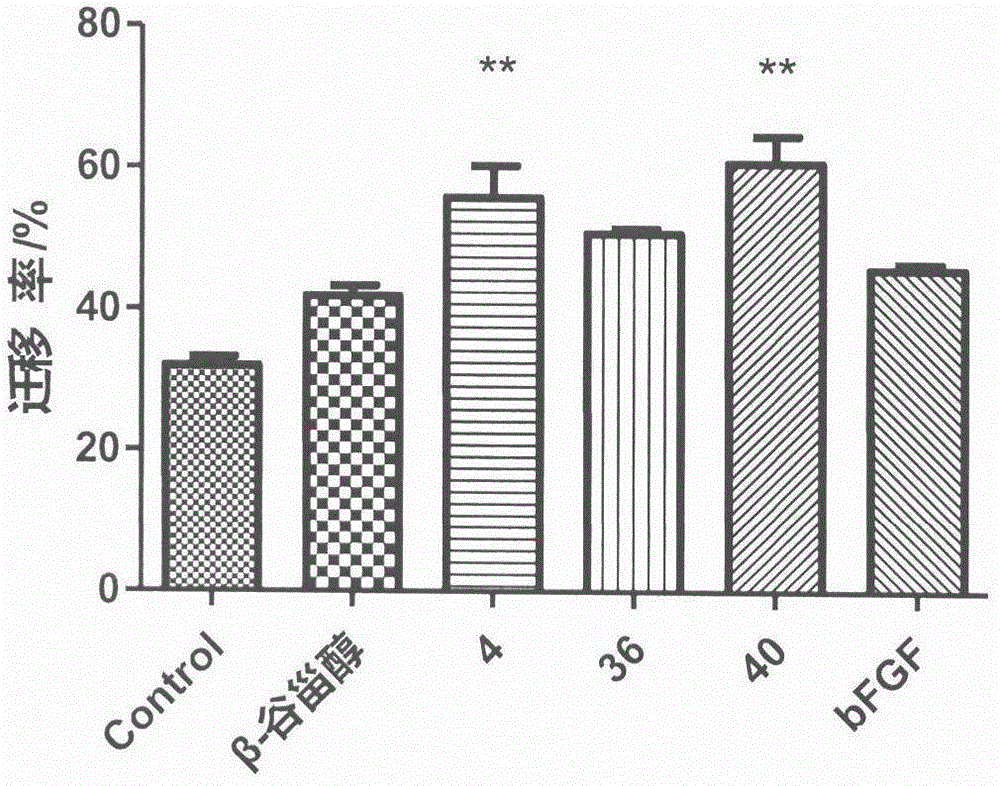
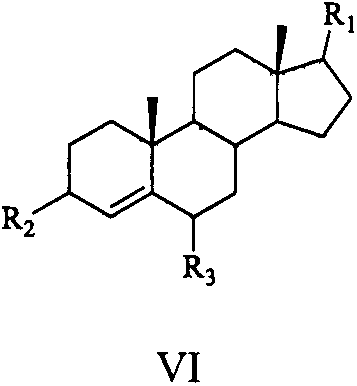
Sterol derivative, and preparation method and application thereof
A technology of sterol derivatives and compounds, applied in the fields of application, steroids, food science, etc., can solve the problems of unreported research on drugs for promoting wound healing, high cholesterol activity, and low toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Sitosta-4-ene-3,6-dione (compound 2)
[0066]Weigh β-sitosterol (compound 1, 1.0 g) and pyridinium chlorochromate (1.0 g), dissolve them in 200 ml of dichloromethane, react at room temperature, and monitor by TLC (thin-layer silica gel plate) until the reaction is complete. After completion of the reaction, cool to room temperature, filter with diatomaceous earth filter cake, and evaporate the solvent under reduced pressure. The residue obtained is subjected to silica gel column chromatography, and the eluent is petroleum ether / ethyl acetate=15:1 (V / V), to obtain light yellow crystal sitoster-4-ene-3,6-dione (compound 2, 854.8mg, yield is 83.1%)
[0067]
[0068] Compound 2: Pale yellow crystals, m.p.ESI-MS m / z: 427.0[M+H]. 1 H NMR (300MHz, CDCl 3 )δ: 0.74 (3H, s, 18-H), 0.83 (3H, d, J=6.0Hz, 26-or 27-H), 0.85 (3H, d, J=6.0Hz, 26-or 27-H ), 0.86(3H, t, J=9.0Hz, 29-H), 0.95(3H, d, J=6.0Hz, 21-H), 1.18(3H, s, 19-H), 2.15-2.18(1H , m, 2-H β ), 2.51-2.57 ...
Embodiment 2
[0069] Example 2 (E)-sitosta-4-ene-3β-oximino-methyl-6-one (compound 3) and (E)-sitosta-3β, 6β-sitosta-methyl-4-ene (compound 4)
[0070] Weigh hydroxylamine hydrochloride (100.0 mg), ethyl acetate (240 μl), sodium hydroxide (126.0 mg), dissolve in 500 μl deionized water, react under ice bath conditions, TLC (thin-layer silica gel plate) monitor until the reaction is complete. After the reaction was completed, methyl bromide (114.1 μl) was added, reacted in an ice bath, and monitored by TLC (thin-layer silica gel plate) until the reaction was complete. After the reaction is complete, extract the by-product with petroleum ether and take the lower layer solution, extract it three times with ethyl acetate, take the ethyl acetate layer, evaporate the solvent under reduced pressure to obtain white crystal methoxyformamide, then add ethanol (10.0ml) . Hydrochloric acid (15 μl), react at 65° C., and monitor by TLC (thin-layer silica gel plate) until the reaction is complete. After ...
Embodiment 3
[0076] Example 3 (E)-sitosta-4-ene-3β-oximino-ethyl-6-ketone (compound 5) and (E)-sitosta-3β, 6β-oximino-ethyl-4-ene ( Compound 6)
[0077] Weigh hydroxylamine hydrochloride (100.0 mg), ethyl acetate (240 μl), sodium hydroxide (126.0 mg), dissolve in 500 μl deionized water, react under ice bath conditions, TLC (thin-layer silica gel plate) monitor until the reaction is complete. After the reaction was completed, ethyl bromide (155.2 μl) was added, reacted in an ice bath, and monitored by TLC (thin-layer silica gel plate) until the reaction was complete. After the reaction is complete, extract the by-product with petroleum ether and take the lower layer solution, extract it three times with ethyl acetate, take the ethyl acetate layer, evaporate the solvent under reduced pressure to obtain white crystal ethoxyformamide, and then add ethanol (10.0ml) , hydrochloric acid (15.0 μl), react at 65° C., and monitor by TLC (thin-layer silica gel plate) until the reaction is complete. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com