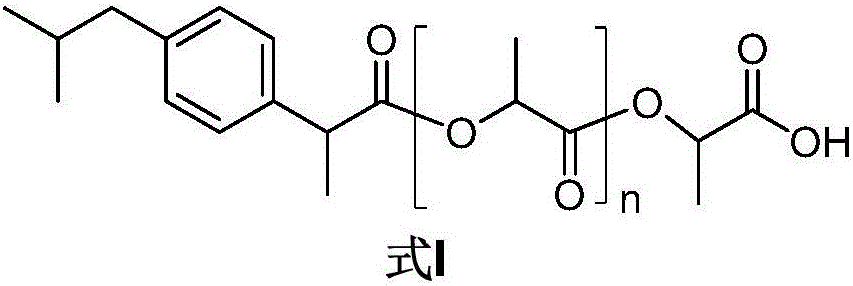
Sustained-release pro-drug with ibuprofen loaded by polylactic acid bonds, method for preparing sustained-release pro-drug by means of direct melt co-polymerization and application of sustained-release pro-drug
A polylactic acid, melt polycondensation technology, applied in pharmaceutical formulations, antipyretics, medical science and other directions, to achieve the effect of simple preparation technology and strong inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Sustained-release Prodrug of Ibuprofen Supported by Polylactic Acid
[0038] (1) Use D, L-LA and ibuprofen as raw materials, mix evenly according to the ratio of substances n(D, L-LA):n(ibuprofen)=120:1, after 140°C, 4000Pa, 8h The intermediate is obtained after the pre-polymerization and water removal treatment;
[0039] (2) Add catalyst SnO (mass percentage is 0.3% of the intermediate), melt and polycondense at a temperature of 160°C and a pressure of 70Pa for 6h; Lactic acid bond loaded ibuprofen sustained-release prodrug;
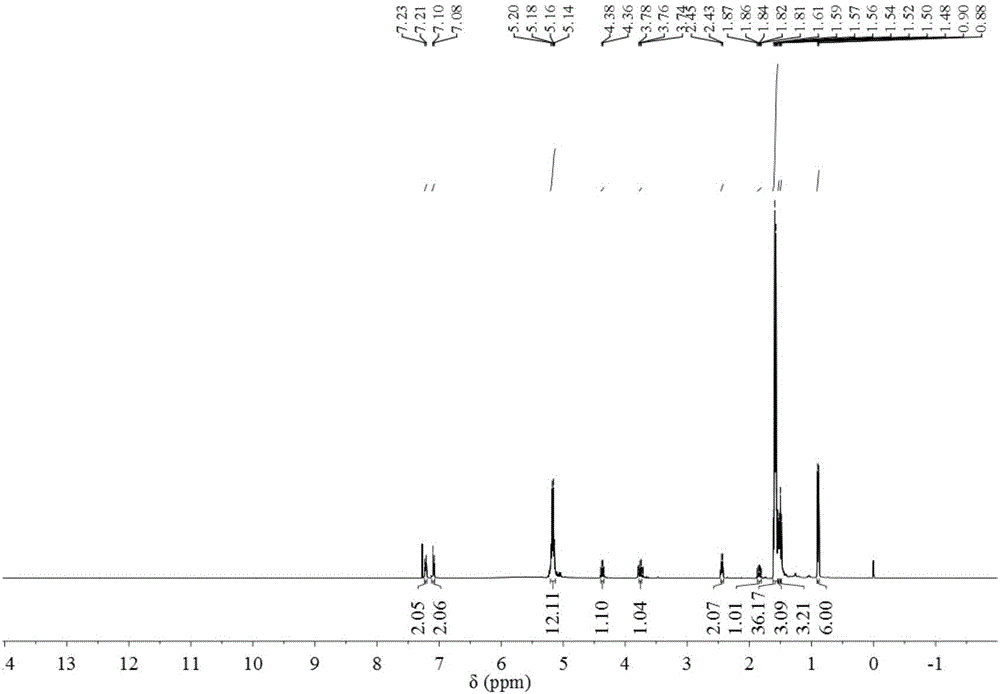
[0040] The structure of the product was confirmed by polymer characterization methods such as infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, and gel permeation chromatography, and the measured intrinsic viscosity [η] = 1.113dL / g. The product structure data are characterized as follows:
[0041] Infrared spectrum related data: 3525.87, O-H stretching vibration absorption peak; 3180.65, C-H stretching vibra...
Embodiment 2
[0044] Synthesis of Sustained-release Prodrug of Ibuprofen Supported by Polylactic Acid
[0045] (1) Use D, L-LA and ibuprofen as raw materials, mix evenly according to the ratio of substances n(D, L-LA):n(ibuprofen)=120:1, after 140°C, 4000Pa, 8h The intermediate is obtained after the pre-polymerization and water removal treatment;
[0046] (2) Add catalyst ZnCl 2 (mass percentage is 0.3% of the intermediate), melting and polycondensation at a temperature of 160° C. and a pressure of 70 Pa for 6 hours; after the reaction, the product was dissolved in chloroform at room temperature, purified by methanol precipitation, and vacuum-dried to obtain a white powder polylactic acid bond-loaded ibuprofen Extended-release prodrugs.
[0047] In the same way as in Example 1, the structure of the product was confirmed by polymer characterization methods such as infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, and gel permeation chromatography, and the measured intr...
Embodiment 3
[0049] Synthesis of Sustained-release Prodrug of Ibuprofen Supported by Polylactic Acid
[0050] (1) Use D, L-LA and ibuprofen as raw materials, mix evenly according to the ratio of substances n(D, L-LA):n(ibuprofen)=120:1, after 140°C, 4000Pa, 8h The intermediate is obtained after the pre-polymerization and water removal treatment;
[0051] (2) Add catalyst SnO (mass percentage is 0.9% of the intermediate), melt and polycondense at a temperature of 160°C and a pressure of 70Pa for 6h; Lactic acid bond loaded ibuprofen sustained release prodrug.
[0052] In the same way as in Example 1, the structure of the product was confirmed by polymer characterization methods such as infrared spectroscopy, hydrogen nuclear magnetic resonance spectroscopy, and gel permeation chromatography, and the measured intrinsic viscosity [η]=0.375dL / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com