Visible light catalyzed saturated carbon-hydrogen bond direct oxidizing method
一种碳氢键、可见光的技术,应用在化学仪器和方法、有机化学方法、有机氧化等方向,能够解决活性低、性质不太稳定过氧化物等问题,达到反应效率高、性质稳定、成本低廉的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
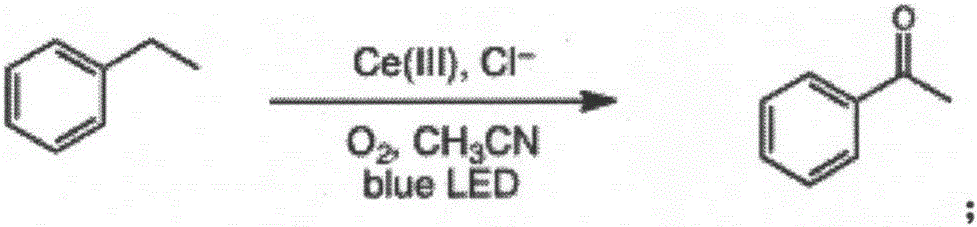
[0021] A method for direct oxidation of saturated carbon-hydrogen bonds catalyzed by visible light, the specific steps are:
[0022] Into 2 mL of ethylbenzene (106 mg, 1 mmol) in acetonitrile solution, bubbling oxygen for 20 min to oxygen saturation, adding 1 mol% cerium complex cerium trichloride (2.4 mg, 0.01 mmol) and 2 mol% additive tetrabutylammonium chloride (5.5mg, 0.02mmol), at room temperature (25°C), under the conditions of blue LED light (wavelength 380nm-550nm), make ethylbenzene and oxygen under the action of cerium complexes and additives Carry out reaction 2h, make the carbon-hydrogen bond oxidation of ethylbenzene obtain oxidation product acetophenone, reaction equation is as follows:
[0023]
[0024] After stopping the reaction, dichloromethane was added to dilute, washed with water and saturated brine successively, dried, and separated by column chromatography to obtain the product acetophenone (108mg, 90% yield), and the data characterized by H NMR were ...
Embodiment 2
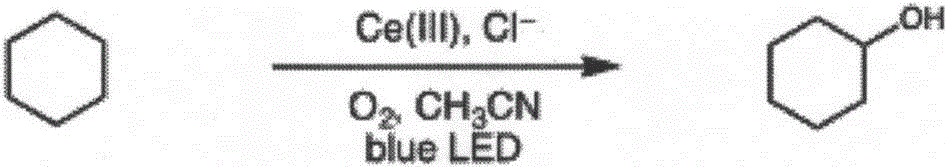
[0026] A method for direct oxidation of saturated carbon-hydrogen bonds catalyzed by visible light, the specific steps are:
[0027] Into 2 mL of cyclohexane (85 mg, 1 mmol) in acetonitrile solution, oxygen was passed through for 20 min to oxygen saturation, and 1 mol% of cerium complex cerium trichloride (2.4 mg, 0.01 mmol) and 2 mol% of additive tetrabutyl chloride were added After ammonium (5.5mg, 0.02mmol), at room temperature (25°C), under the condition of blue LED light (wavelength 380nm-550nm), make cyclohexane and oxygen in the complex of cerium and additives Under the effect, carry out reaction 5h, make the carbon-hydrogen bond oxidation of cyclohexane obtain oxidation product cyclohexanol, and reaction equation is as follows:
[0028]
[0029] After stopping the reaction, dichloromethane was added to dilute, washed with water and saturated brine successively, dried, and separated by column chromatography to obtain the product cyclohexanol (88 mg, 88% yield). The ...
Embodiment 3
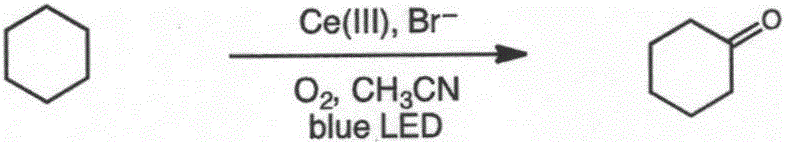
[0031] A method for direct oxidation of saturated carbon-hydrogen bonds catalyzed by visible light, the specific steps are:
[0032] Oxygen was passed through 2 mL of cyclohexane (85 mg, 1 mmol) in acetonitrile solution for 20 min to oxygen saturation, and 2 mol % cerium complex cerium nitrate (8.7 mg, 0.02 mmol) and 4 mol % additive tetrabutylammonium bromide ( 12.9mg, 0.04mmol), at room temperature (25°C), under the condition of blue LED light (wavelength 380nm-550nm) irradiation, make cyclohexane and oxygen under the action of cerium complexes and additives Carry out reaction 48h, make the carbon-hydrogen bond oxidation of cyclohexane obtain oxidation product cyclohexanone, and reaction equation is as follows:
[0033]
[0034] After stopping the reaction, dichloromethane was added to dilute, washed with water and saturated brine successively, dried, and separated by column chromatography to obtain the product cyclohexanone (80 mg, 82% yield). The data characterized by ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com