In-vivo in-situ drug-loading hydrogel carrier as well as preparation method and applications thereof
A hydrogel and carrier technology, applied in prosthesis, medical science, tissue regeneration, etc., can solve the problems of poor fusion rate, no bionic artificial bone marrow stent, etc., and achieve the effect of improving spinal fusion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of in situ drug-loaded hydrogel carrier PLGA-PEG-PLGA in vivo
[0036] The following raw materials: polyethylene glycol PEG (1500) (Aldrich-Sigma Co.), lactide DL-lactide (LA) (Purac), glycolide (GA) (Purac), stannous octoate (99%, Aldrich-SigmaCo.), and anhydrous toluene and dichloromethane are all commercially available.
[0037] Add 20g of PEG (1500) with double-terminated hydroxyl groups in a 250ml flask, heat the oil bath to 120°C, remove water under reduced pressure for 4 hours, then add 48.8g of monomer lactide (LA) and 12.2g of glycolide ( GA) with a molar ratio of 4:1, heat in a vacuum environment until it is completely melted, add 150 microliters of stannous octoate anhydrous toluene solution to catalyze, remove toluene under reduced pressure for 1 hour, heat the oil bath to 150°C and continue the reaction under argon 6 hours.
[0038] After the reaction was completed, the temperature was lowered to 100°C, and unreacted monomers and low...
Embodiment 2
[0039] Embodiment 2 Hydrogel Carrier Number Average Molecular Weight, Molecular Weight Distribution, Determination of Transformation Temperature
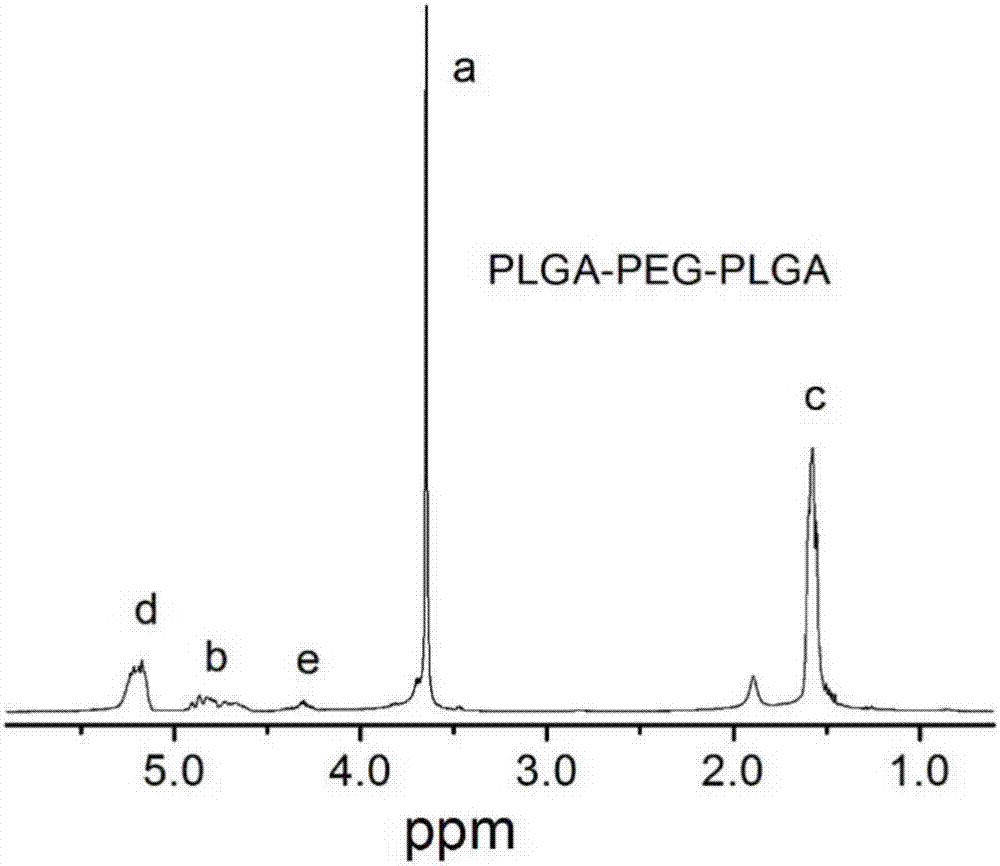
[0040] 1. The actual number-average molecular weight obtained by calculating the PLGA-PEG-PLGA hydrogel carrier prepared in Example 1 according to NMR is 1744-1500-1744, LA:GA=4:1, such as figure 1 shown.
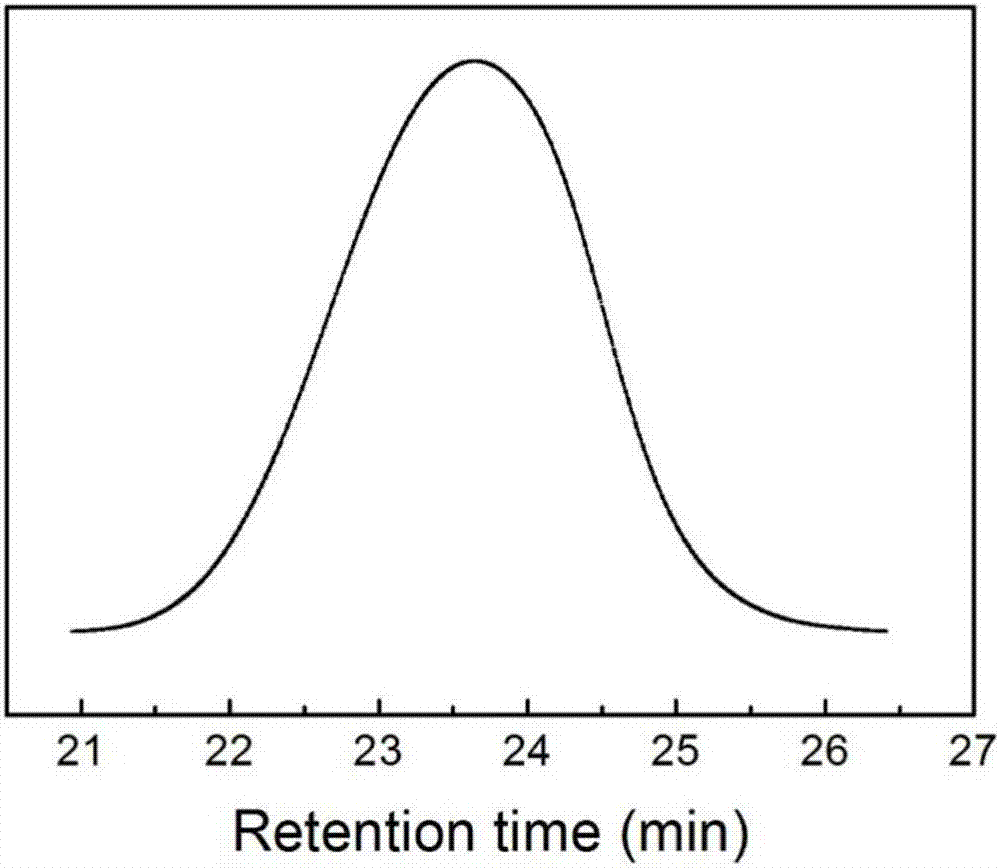
[0041] 2. The PLGA-PEG-PLGA of embodiment 1 is analyzed and calculated by GPC as a homogeneous substance, its number average molecular weight Mn: 5956, weight average molecular weight Mw: 8732, molecular weight distribution coefficient D: 1.26, as figure 2 shown.
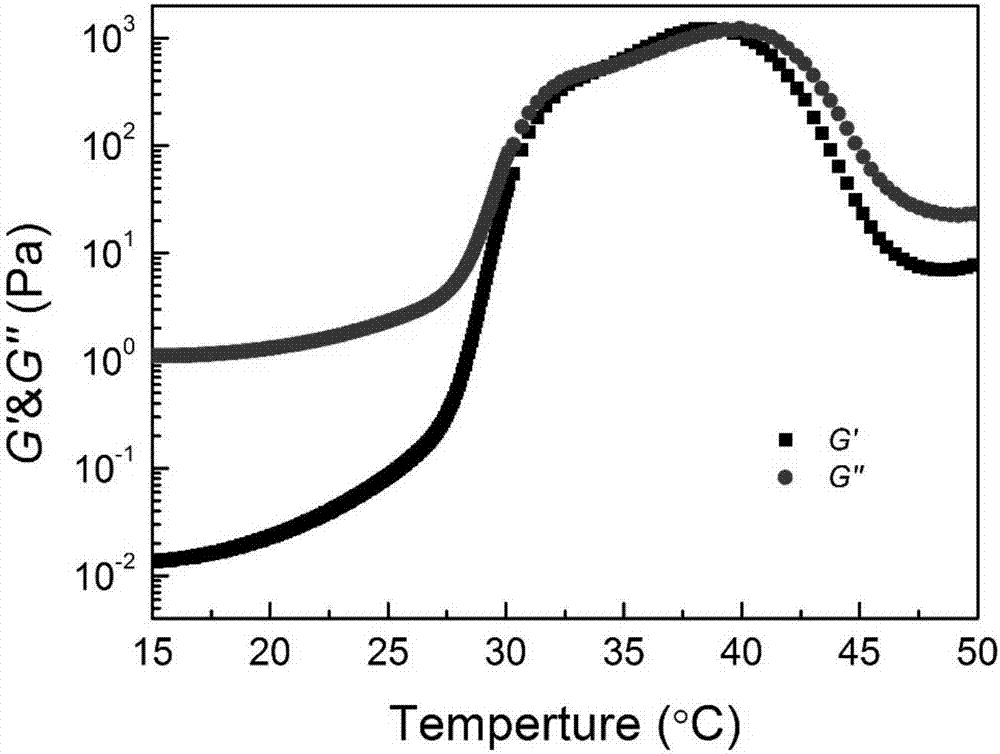
[0042] 3. Configure a 25wt% solution of PLGA-PEG-PLGA with physiological saline, and its rheological diagram is as follows image 3 As shown, it can be seen that when the heating rate reaches about 27-30°C at a rate of 0.5°C / min, the hydrogel begins to change phases, and when it exceeds 30°C, it basically changes from a flowing sol state to a solid gel state (such a...
Embodiment 3
[0043] Example 3 Preparation of rhBMP2 / rhVEGF165-loaded gel PLGA-PEG-PLGA
[0044] The drug-loaded hydrogel carrier PLGA-PEG-PLGA prepared in Example 1 was dissolved in physiological saline to prepare 40ml of a 25wt% hydrogel solution, which was sterilized by 7kGy irradiation (the second military medical university irradiated Center) and put it in a refrigerator at 4°C for 24 hours until it becomes a homogeneous solution state. At room temperature (below 25°C), use a 5ml sterile syringe to extract 14ml of hydrogel solution containing 500ug of rhVEGF165 in a sterile ultra-clean bench In the reagent bottle, the reagent bottle mixed with hydrogel and rhVEGF was placed in a mixer and mixed for 5 minutes to prepare a mixture V solution containing 36ug / ml of VEGF;
[0045] Inject 14ml of the hydrogel solution into a reagent bottle containing 5mg of rhBMP-2 to prepare a mixture B solution containing rhBMP-2 at a concentration of 360ug / ml;
[0046] Take a sterile centrifuge tube (15m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com