All-solid-state lithium-sulfur battery
A lithium-sulfur battery, all-solid-state technology, used in batteries, secondary batteries, battery electrodes, etc., can solve the problems of flying shuttle effect, large interface contact resistance, and reduced Coulomb efficiency, and achieve the advantages of large-scale production and interface contact. Small impedance and the effect of improving Coulombic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
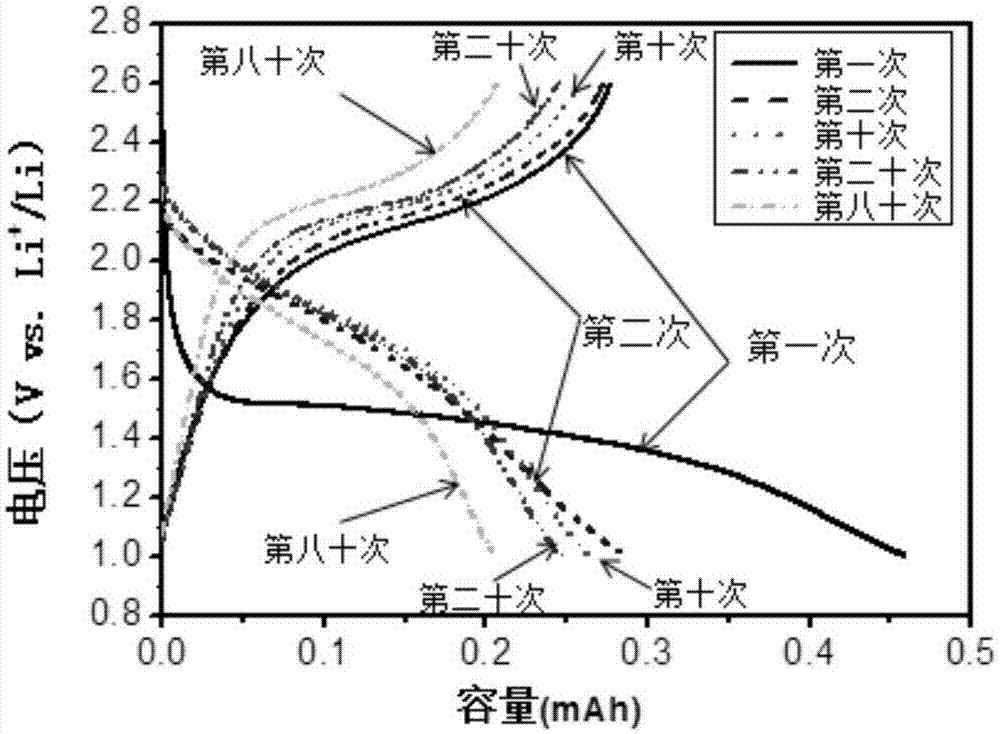
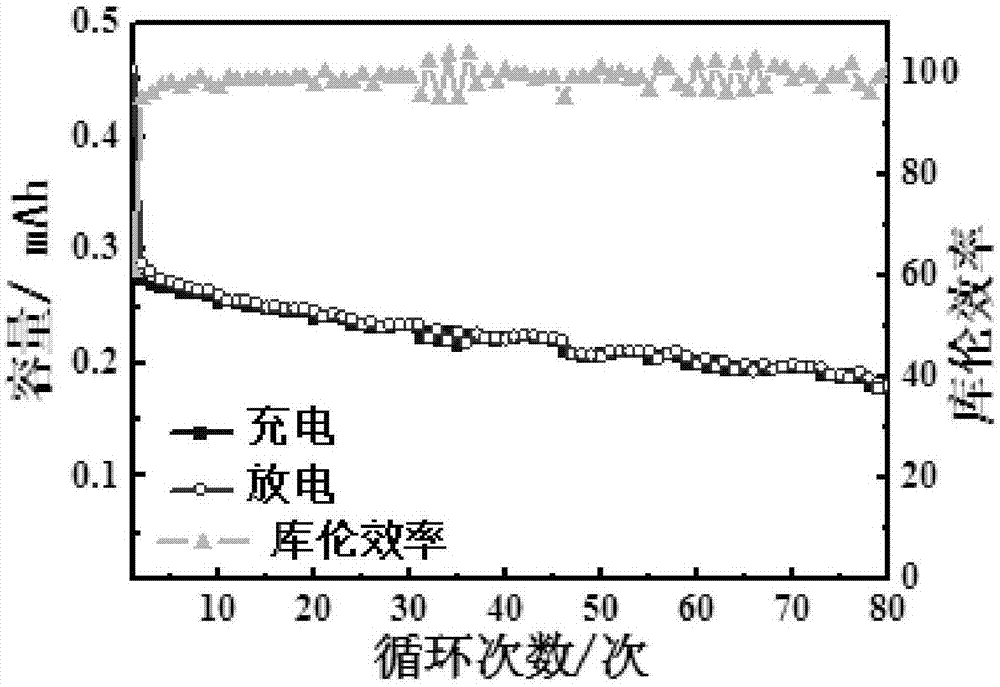
[0042] Soak the perfluorosulfonic acid-polytetrafluoroethylene membrane in 1M LiOH aqueous solution at 80°C for 12 hours, then wash it with solvent for 3 to 10 times to remove the LiOH on the membrane surface, and then dry it at 100°C for 2 hours. Vacuum drying at 60°C for 48 hours to obtain a lithiated perfluorosulfonic acid-polytetrafluoroethylene membrane. Using NMP as solvent, a lithiated perfluorosulfonic acid-polytetrafluoroethylene polymer emulsion with a mass fraction of 5% was prepared at 80°C. Sulfurized polyacrylonitrile, acetylene black, and lithiated perfluorosulfonic acid-polytetrafluoroethylene polymer are weighed at a mass ratio of 6:2:2 as the positive electrode active material, mixed and ground with NMP as a solvent, and then coated on an aluminum foil and dried overnight in a vacuum oven at 55°C to obtain a sulfur cathode. The lithiated perfluorosulfonic acid-polytetrafluoroethylene membrane and the sulfur positive electrode were hot-pressed at 120 °C and 0...
Embodiment 2
[0044] Soak the polytrifluorostyrene sulfonic acid membrane in 2M LiOH in DMSO solution at 100°C for 12 hours, then wash it with a solvent for 3 to 10 times to remove LiOH on the membrane surface, and then dry it at 100°C for 6 hours. Then vacuum-dry at 100°C for 48 hours to obtain a lithiated polytrifluorostyrene sulfonic acid membrane. Using DMF as a solvent, a lithiated polytrifluorostyrene sulfonic acid polymer emulsion with a mass fraction of 10% was prepared at 60°C. Weigh the positive electrode active materials iron sulfide, Super-P, and lithiated polytrifluorostyrene sulfonic acid polymer at a mass ratio of 5:1:4, mix and grind them evenly with DMF as a solvent, and coat them on carbon-coated aluminum foil , dried overnight in a vacuum oven at 55°C to obtain a sulfur cathode. The lithiated polytrifluorostyrene sulfonic acid membrane and the sulfur positive electrode were hot-pressed at 110 °C and 1 MPa pressure for 3 min to obtain an integrated positive electrode and ...
Embodiment 3
[0046] The polydifluorostyrene sulfonic acid membrane was soaked in a mixed solution of 1M LiOH ethanol and water (volume ratio 1:1) at 80°C for 12 hours, then washed with solvent for 3 to 10 times to remove LiOH on the membrane surface, and then placed on Blast drying at 80°C for 6 hours, and then vacuum drying at 40°C for 48 hours to obtain a lithiated polydifluorostyrene sulfonic acid membrane. Using DMAc as a solvent, a lithiated poly(difluorostyrene sulfonic acid) polymer emulsion with a mass fraction of 4% was prepared at 90°C. The positive electrode active material sublimated sulfur selenium solid solution, ketjen black, and lithiated polydifluorostyrene sulfonic acid polymer were weighed at a mass ratio of 4:3:3, mixed and ground with DMAc as a solvent, and then coated on copper foil and dried overnight in a vacuum oven at 55°C. The lithiated polydifluorostyrene sulfonic acid membrane and the sulfur positive electrode were hot-pressed at 100°C and 2 MPa pressure for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com