Amphiphilic lipid-droplet fluorescent probe with super-high selectivity and application thereof
A fluorescent probe and selective technology, applied in the lipid droplet fluorescent probe and its application field, can solve the problems of difficulty in imaging lipid droplets with high selectivity, hindering further research on lipid droplets, and failing to meet research needs, and achieve rapid Dyeing ability, good light stability, fast dyeing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 4-(3'-bromopropylamino)-7-benzofurazan (1)
[0033] In a flask, 4-chloro-7-nitrophenylfurazan (2g, 10mmol) was dissolved in methanol, stirred for 15 minutes, then added with 3-bromopropylamine (2.19g, 10mmol), and reacted at room temperature for 8 hours. After the reaction, it was extracted with dichloromethane and washed with water. Dry with anhydrous sodium sulfate. Finally, a mixture of petroleum ether and ethyl acetate was used for column chromatography to obtain the final product.
[0034] 1H NMR (300MHz, DMSO-d6): δ (ppm) 9.54 (t, J = 5.40Hz, 1H), 8.54 (d, J = 9.00Hz, 1H), 6.45 (d, J = 8.70Hz, 1H), 3.64(m,4H),2.23(m,2H).
[0035] Synthesis of 1-(3'-(7'-nitrobenzofurazan-4'-)aminopropyl)-2,3,3-trimethylindole bromide (NII)
[0036] The synthesized compound (1) (0.3g, 1mmol) was mixed with 2,3,3-trimethylindole (240Ml, 1.5mmol), and heated to reflux with ethanol as a solvent. After 24 hours, the reaction was completed and cooled to room temperatur...
Embodiment 2
[0038] Embodiment 2: the cultivation of immortalized cancer cell HeLa
[0039] All HeLa cell lines were stored at 37°C, 5% CO 2 CO 2 cultured in an incubator. HeLa cells were cultured adherently in H-DMEM medium containing 10% fetal bovine serum and 1% double antibody.
[0040] When the cells grow to the logarithmic phase, culture the slices: ① Soak the coverslips in absolute ethanol for 30 minutes, dry them with an alcohol lamp, and place them in a disposable 35mm culture dish; ② Wash the cells in the 100mL cell bottle with PBS. Three times, digest with 1mL 0.25% trypsin for 3-5 minutes, pour out the medium carefully, add a small amount of fresh medium and pipette evenly, after counting the cells, leave cells with a suitable density, and add the medium to the required volume (The final concentration of control cells was 1×10 5 ), inoculated into a petri dish containing a coverslip, and placed in CO 2 Cultured in an incubator to allow the cells to grow on the sheet.
Embodiment 3
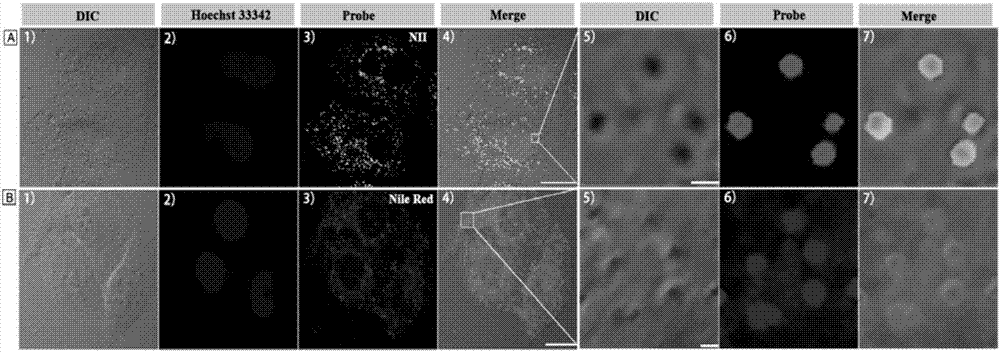
[0041] Example 3: Confocal fluorescence microscopy experiments of lipid droplets in HeLa cells stained by NII and Nile red
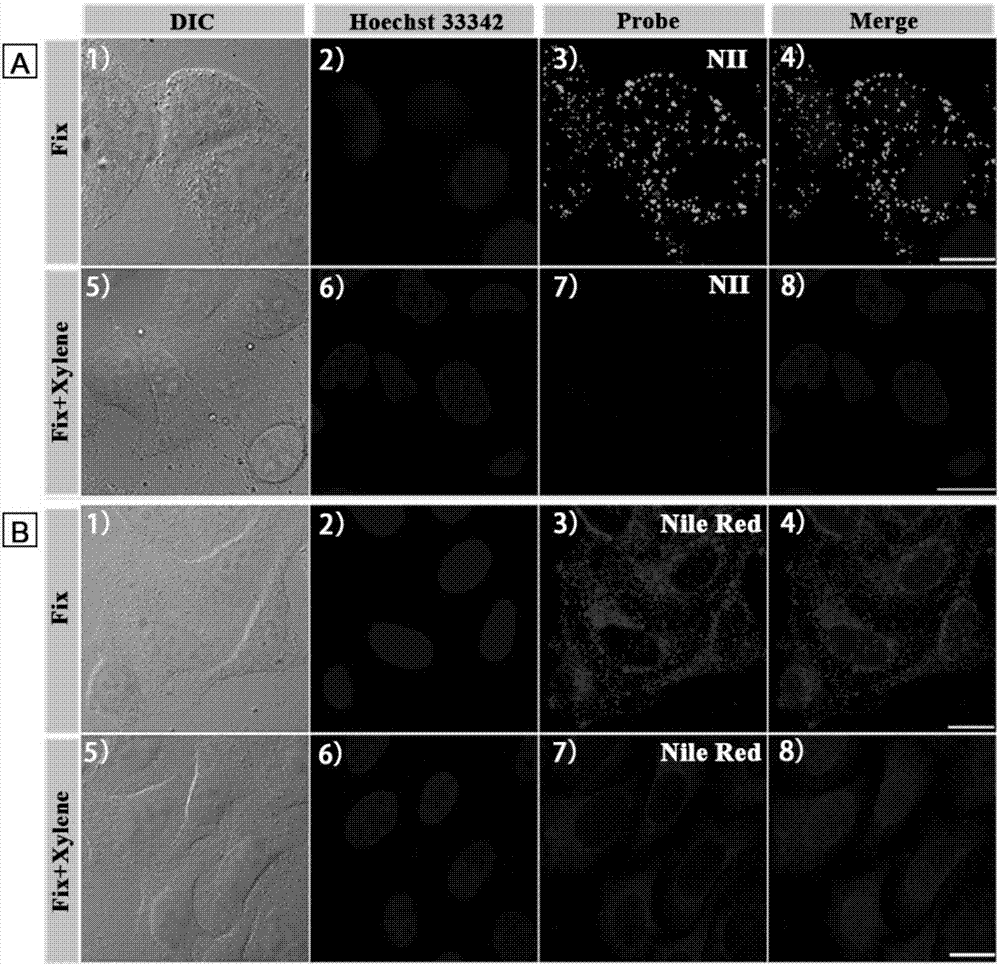
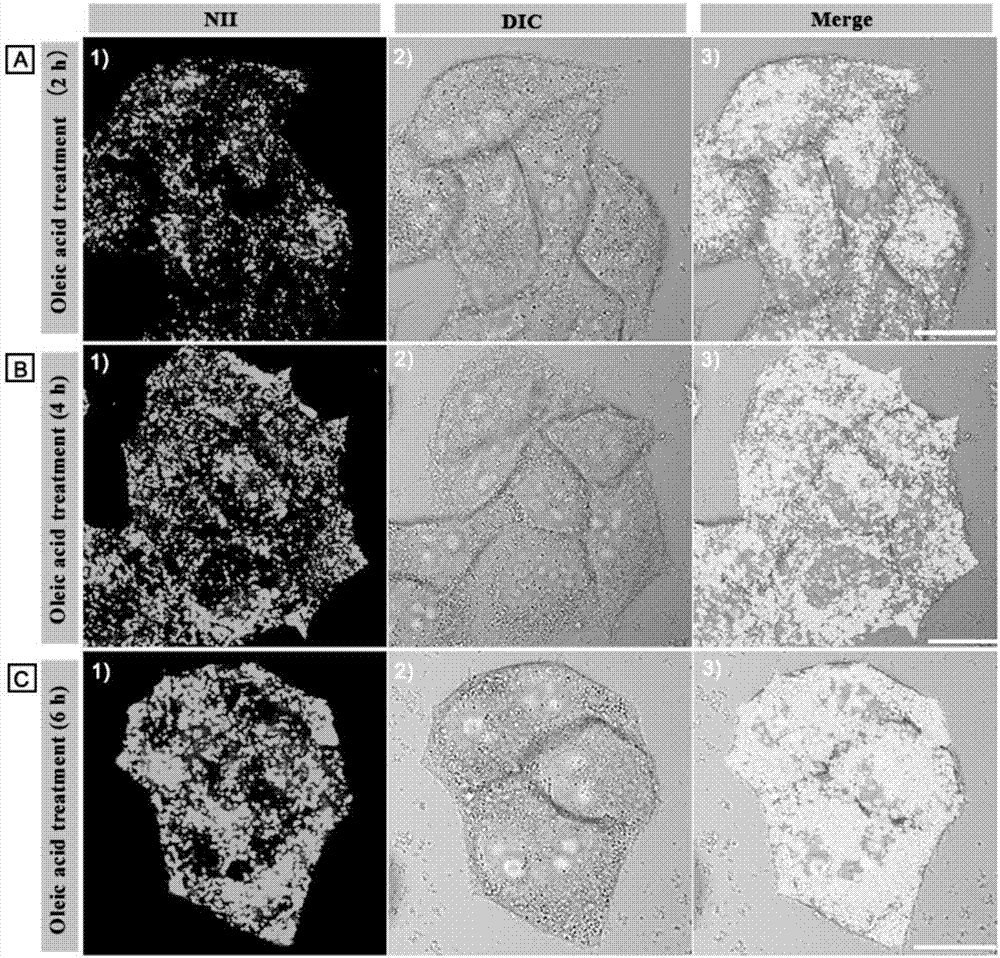
[0042] The attached cell slides were first incubated with nuclear dye Hoechst 33342 (5 μM) for 30 min, washed twice with PBS, and then stained with 4 μM NII for 2 min or 5 μM Nile red for 20 min at room temperature. The cell slides were taken out, and the cell growth side was covered on the glass slide, and observed under a confocal fluorescence microscope. It was found that the lipid droplets in the cells were clearly stained by NII, spherical in shape, and mainly distributed in the cytoplasm (not stained by NII). Hoechst colored area). However, Nile red stains most intracellular structures in addition to dots. Therefore, the amphiphilic probe NII of the present invention is a lipid droplet probe with ultrahigh selectivity, which can give a fluorescence picture of lipid droplets without background noise.
[0043] see results figure 1 . Confocal fluo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com