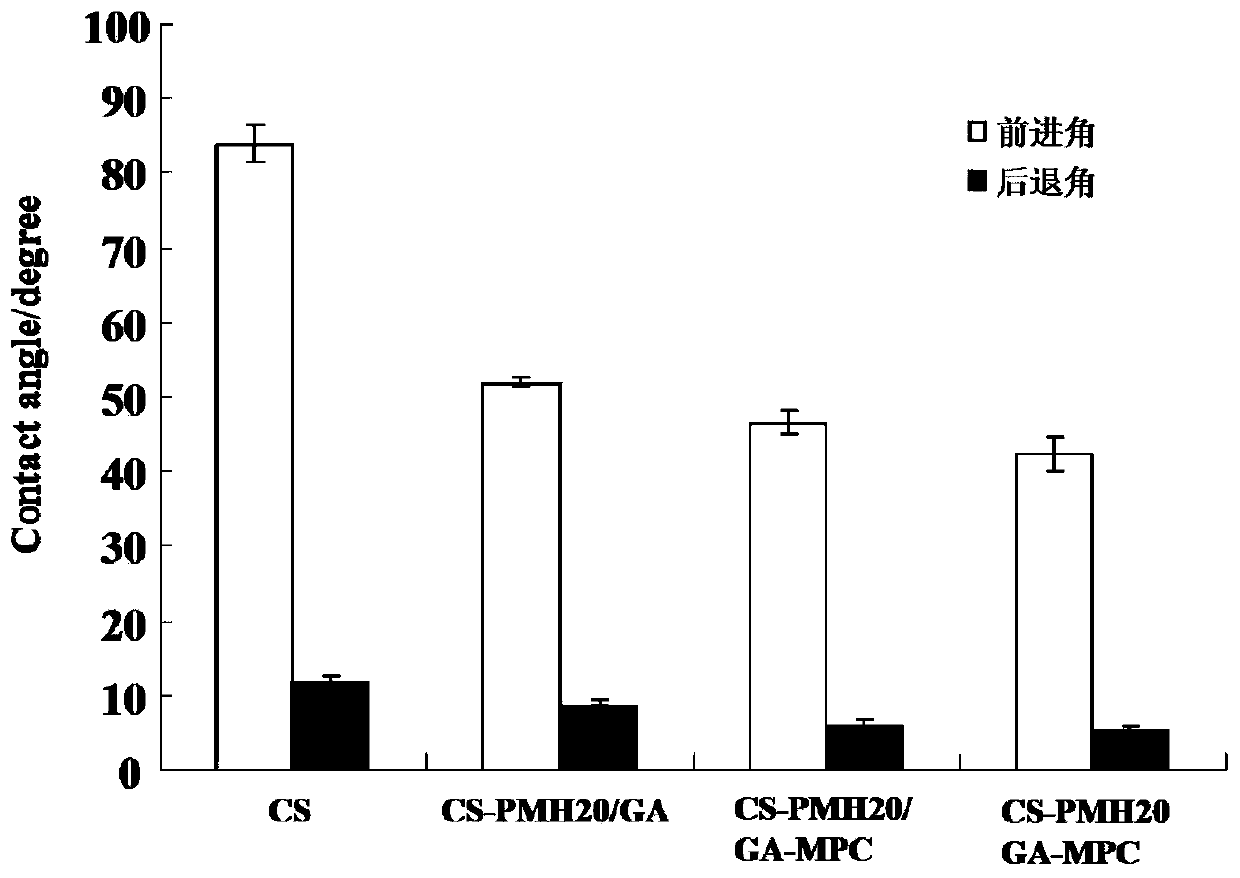
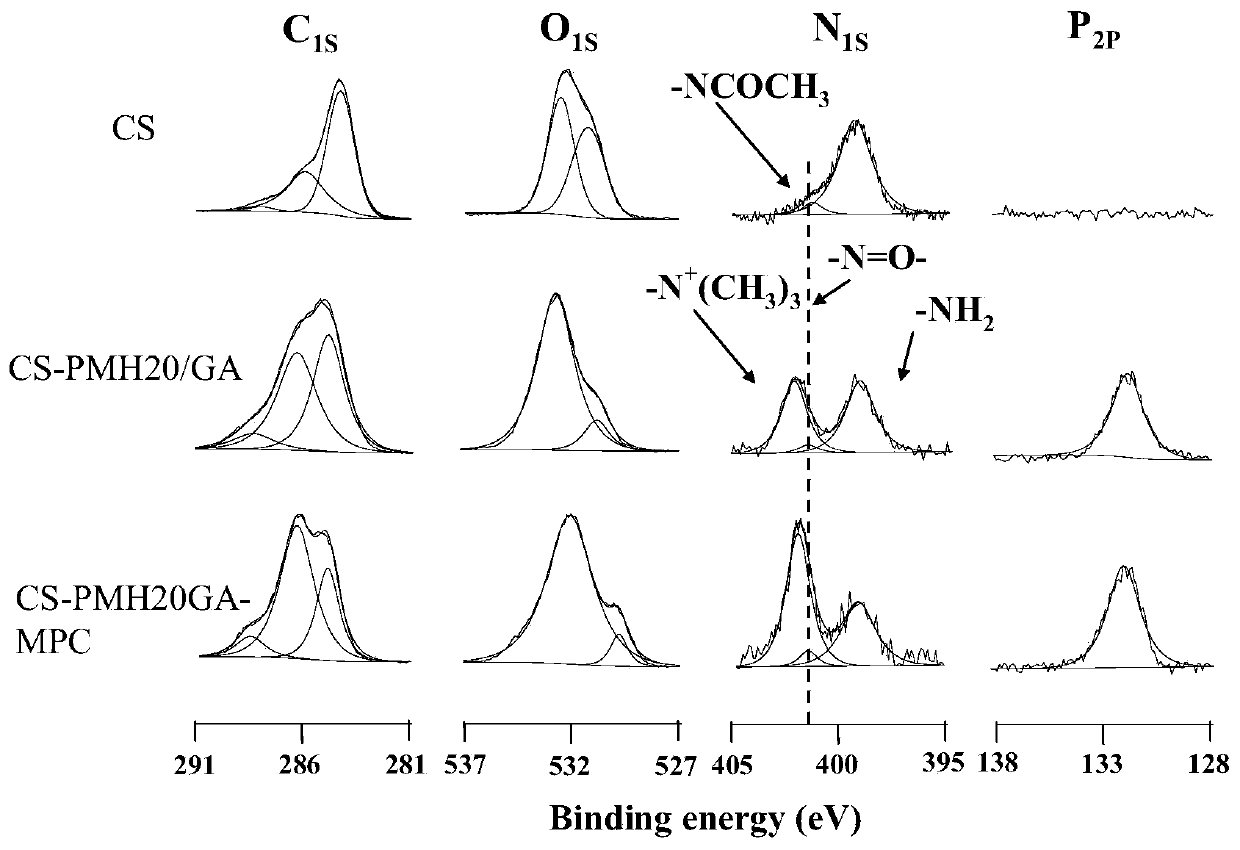
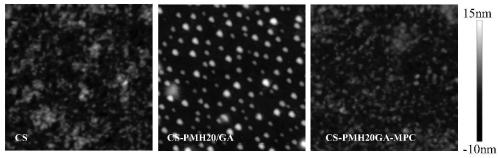
A method for densification of amino-containing phosphorylcholine polymer and glutaraldehyde biomimetic coating
A phosphorylcholine and polymer technology, which is applied in the field of phosphorylcholine polymer and glutaraldehyde biomimetic coating densification, can solve application difficulties, it is difficult to simulate the outer membrane structure of cells, and phosphorylcholine on the coating surface can be solved. problems such as low density of base groups, to achieve the effects of mild conditions, simple preparation methods and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Weigh 16mmol 2-methacryloyloxyethylphosphorylcholine and 4mmol 2-aminoethyl methacrylate hydrochloride, use 0.1mmol potassium persulfate as initiator, under nitrogen protection, 70°C Polymerization was performed for 24 hours, dialyzed after the reaction, and then freeze-dried at -50°C to obtain amino group-containing phosphorylcholine polymer PMH20 (20 represents 20% molar ratio of amino group vinyl monomer in the polymer synthesis process).
[0033] Using a 400MHz NMR instrument to D 2 O is the hydrogen NMR of the solvent test polymer. There is no peak at 5-7ppm, indicating that there is no residual monomer in the resulting copolymer, and the polymer has been successfully synthesized, with 3.28ppm being -N + (CH 3 ) 3 Characteristic peaks, 0.9-2.2ppm are the peaks of methylene and side chain methyl groups on the main chain to calculate the polymer composition, and it can be known that the polymer composition is basically consistent with the feed ratio.
[0034] ...
Embodiment 2
[0044] Weigh 17mmol 2-methacryloyloxyethylphosphorylcholine and 3mmol 2-aminoethyl methacrylate hydrochloride, use 0.1mmol potassium persulfate as the initiator, and polymerize at 70°C under nitrogen protection After 24 hours, the reaction was dialyzed, and then freeze-dried at -50°C to obtain an amino group-containing phosphorylcholine polymer.
[0045] The amino group-containing phosphorylcholine polymer and glutaraldehyde in this embodiment are dissolved in an ethanol solvent in a molar ratio of 100:5 according to the amino group and the aldehyde group. After mixing uniformly, the concentration of the amino group-containing phosphorylcholine polymer is 0.5mg / mL, coating volume 10μl / cm 2 / surface is dried on the surface of chitosan material, and then soaked in 20mL, 5mg / mL methanol solution of 2-methacryloyloxyethylphosphorylcholine, reacted at 20°C for 12h, and then soaked in distilled water Treat at 80 degrees for 12 hours, and then wash with a large amount of methanol a...
Embodiment 3
[0047] Weigh 12mmol 2-methacryloyloxyethyl phosphorylcholine and 8mmol 2-aminoethyl methacrylate hydrochloride, use 0.1mmol potassium persulfate as the initiator, and polymerize at 70°C under nitrogen protection After 24 hours, the reaction was dialyzed, and then freeze-dried at -50°C to obtain an amino group-containing phosphorylcholine polymer.
[0048] The amino group-containing phosphorylcholine polymer and glutaraldehyde in this embodiment are dissolved in methanol solvent in a molar ratio of 100:15 according to the amino group and the aldehyde group. After mixing uniformly, the concentration of the amino group-containing phosphorylcholine polymer is 1.5mg / mL, coating volume 10μl / cm 2 / surface is dried on the surface of chitosan material, and then soaked in 25mL, 15mg / mL methanol solution of 2-methacryloyloxyethylphosphorylcholine, reacted at 30°C for 8h, and then soaked in distilled water Treat at 85 degrees for 10 hours, and then wash with a large amount of methanol a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com