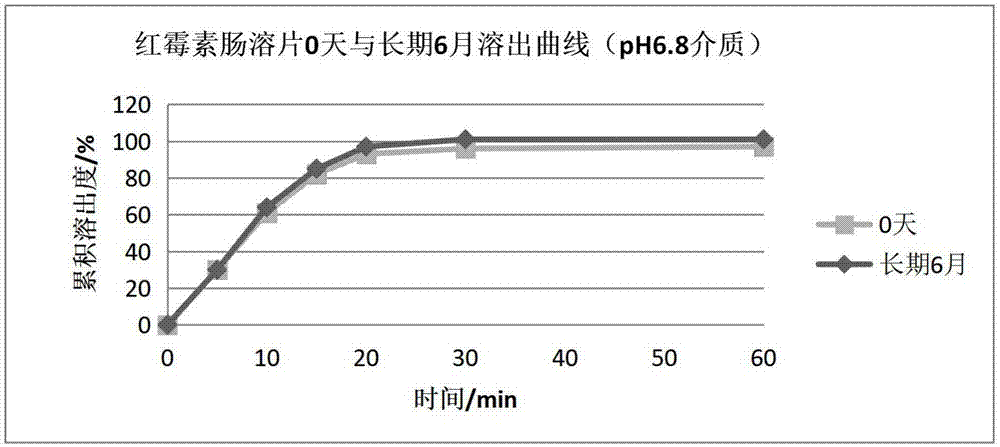
Erythromycin enteric-coated tablet and preparation method thereof
A technology of erythromycin and enteric-coated tablets, which is applied in the field of medicine, can solve problems such as unstable release, poor bioavailability, and reduced release, and achieve quality assurance and curative effect, uniform coating layer, and stable release. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
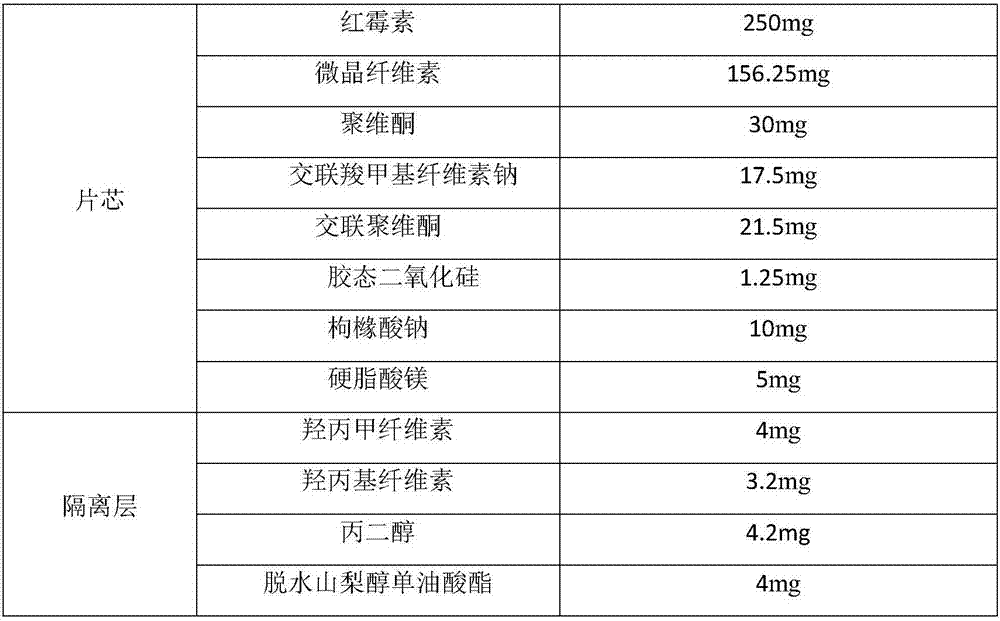
[0035] The prescription is as follows:
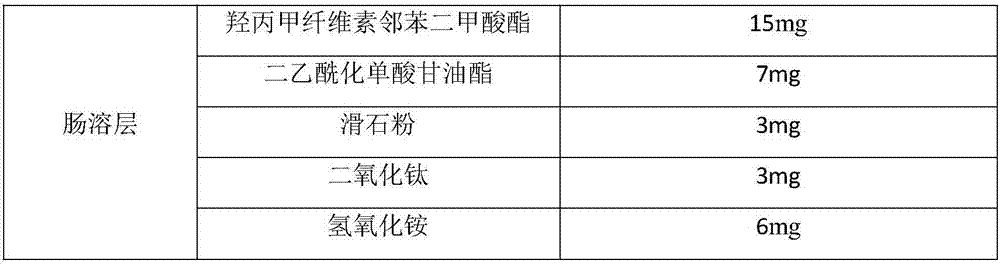
[0036]
[0037]
[0038] Preparation:
[0039] (1) Prepare the adhesive: dissolve povidone with purified water, and then add the prescribed amount of sodium citrate to dissolve.
[0040] (2) Pour erythromycin and microcrystalline cellulose into a wet granulator and mix evenly according to the prescription ratio, add the binder prepared in (1) according to the prescription ratio, and stir to make wet granules.
[0041] (3) Dry the prepared wet granules in a fluidized dryer until the moisture content is 4.5% to 6.5%, and then granulate with a granulator.
[0042] (4) Add croscarmellose sodium, crospovidone, colloidal silicon dioxide, magnesium stearate and the granules prepared in (3) into the mixer according to the prescription ratio and mix to obtain enteric-coated tablets Uniform particles.
[0043] (5) Tablet compression, using a tablet press to compress tablets to obtain tablet cores.
[0044] Carry out the package isolatio...
Embodiment 2
[0050] The prescription is as follows:
[0051]
[0052]
[0053] Preparation:
[0054] (1) Prepare the adhesive: dissolve povidone with purified water, and then add the prescribed amount of sodium citrate to dissolve.
[0055] (2) Pour erythromycin and microcrystalline cellulose into a wet granulator and mix evenly according to the prescription ratio, add the binder prepared in (1) according to the prescription ratio, and stir to make wet granules.
[0056] (3) Dry the prepared wet granules in a fluidized dryer until the moisture content is 4.5% to 6.5%, and then granulate with a granulator.
[0057] (4) Add croscarmellose sodium, crospovidone, colloidal silicon dioxide, magnesium stearate and the granules prepared in (3) into the mixer according to the prescription ratio and mix to obtain enteric-coated tablets Uniform particles.
[0058](5) Tablet compression, using a tablet press to compress tablets to obtain tablet cores.
[0059] Package isolation layer:
[00...
Embodiment 3
[0065] The prescription is as follows:
[0066]
[0067] Preparation:
[0068] (1) Prepare the adhesive: dissolve povidone with purified water, and then add the prescribed amount of sodium citrate to dissolve.
[0069] (2) Pour erythromycin and microcrystalline cellulose into a wet granulator and mix evenly according to the prescription ratio, add the binder prepared in (1) according to the prescription ratio, and stir to make wet granules.
[0070] (3) Dry the prepared wet granules in a fluidized dryer until the moisture content is 4.5% to 6.5%, and then granulate with a granulator.
[0071] (4) Add croscarmellose sodium, crospovidone, colloidal silicon dioxide, magnesium stearate and the granules prepared in (3) into the mixer according to the prescription ratio and mix to obtain enteric-coated tablets Uniform particles.
[0072] (5) Tablet compression, using a tablet press to compress tablets to obtain tablet cores.
[0073] Pack isolation layer and enteric coating t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com