Preparation method of propiolic acid compounds
A technology of propynoic acid and compound is applied in the field of preparation of propynoic acid compounds, and can solve the problems of difficult handling, expensive transition metal catalyst, expensive alkali TBD and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
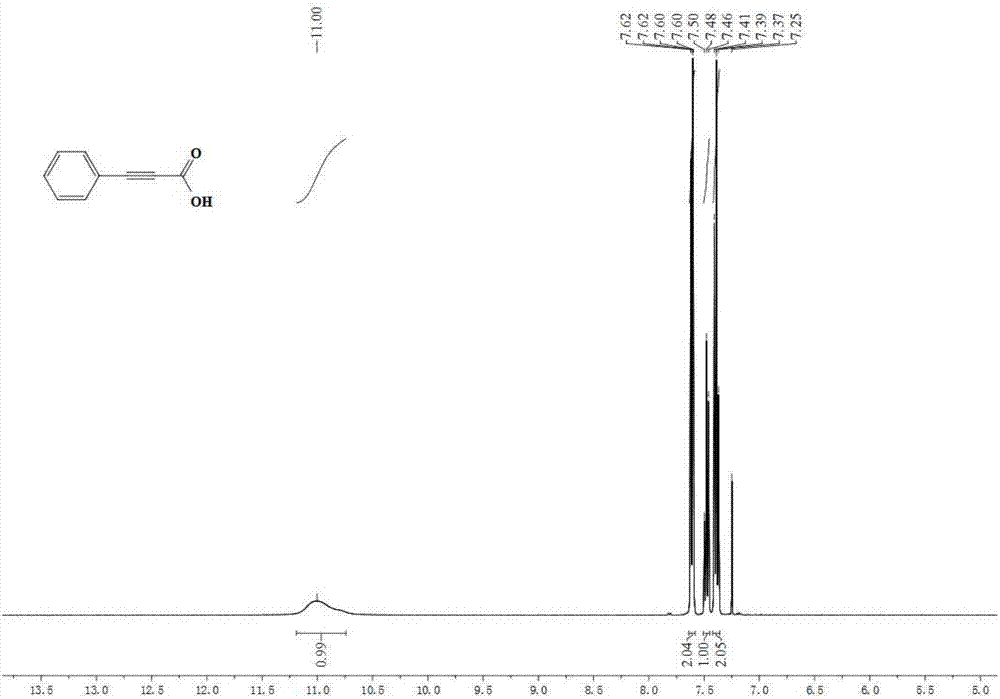
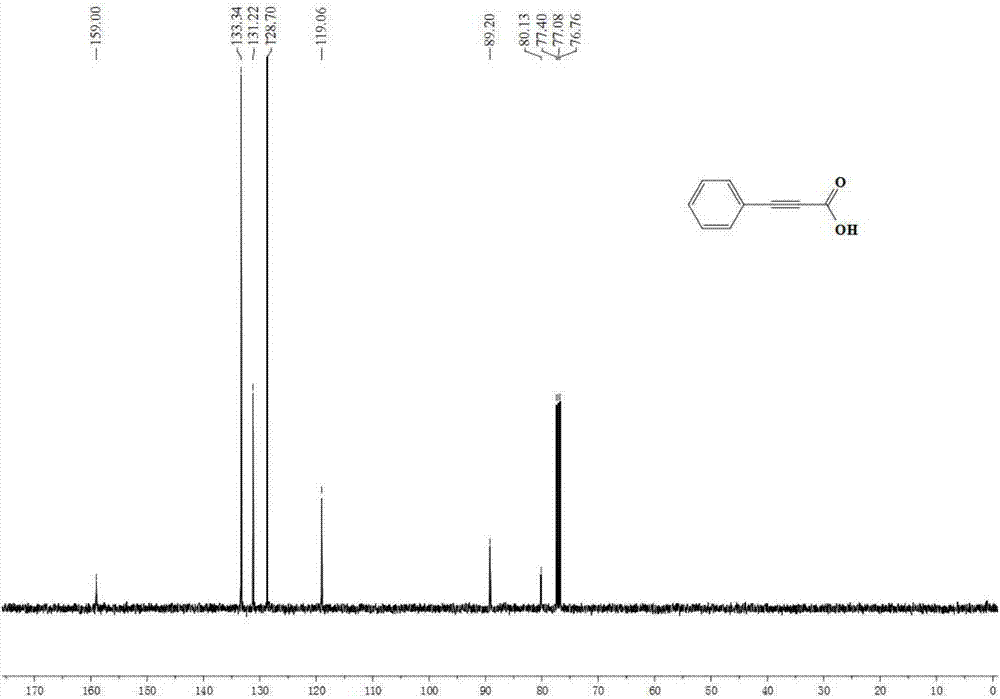
[0039] Embodiment 1: the synthesis of phenylpropiolic acid
[0040]
[0041] Accurately measure potassium carbonate (552mg, 4mmol, 400mol%), tetrabutylammonium acetate (451.5mg, 1.5mmol, 150mol%), refined acetonitrile (5.0mL), phenylacetylene (102mg, 1mmol) in the glove box , were added to the 25mL reaction kettle in turn, and the glove box was filled with CO 2 (0.1MPa). The reaction kettle was closed and placed in an oil bath at 90°C for 4 hours. After the reaction was finished, the reactor was slowly cooled to room temperature, and then the remaining gas was slowly released. The remaining reaction solution in the reaction kettle was transferred to a one-necked bottle, acidified to PH=1 by adding 1M hydrochloric acid, extracted with ethyl acetate, collected the organic phase, removed the solvent in vacuo, and separated through a silica gel column (eluent: petroleum ether / ethyl acetate =6 / 1) to obtain 124 mg of phenylpropiolic acid with a yield of 85%. 1 H NMR (400MHz, ...
Embodiment 2
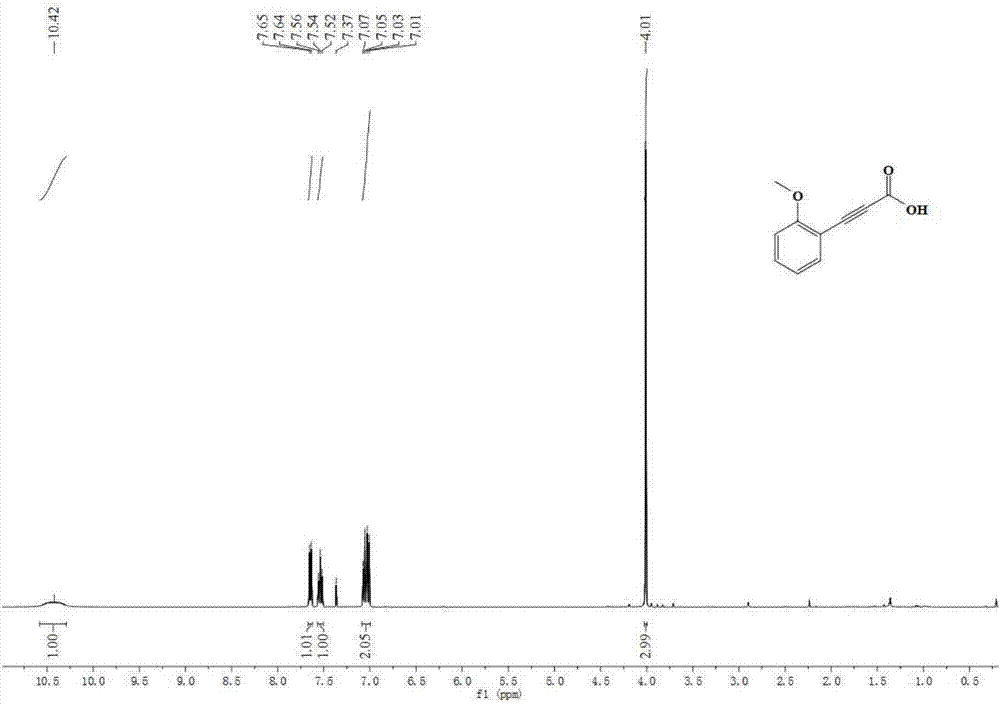
[0042] Embodiment 2: the synthesis of 2-methoxyphenylpropynoic acid
[0043]
[0044] Accurately measure sodium carbonate (312mg, 3mmol, 300mol%), tetrabutylammonium nitrate (304mg, 1mmol, 100mol%), refined THF (5.0mL), 2-methoxyphenylacetylene (132mg , 1mmol), were sequentially added to a 25mL reaction kettle, and the glove box was filled with CO 2 (4MPa). The reactor was closed and placed in an oil bath at 30°C for 36 hours. After the reaction was finished, the reactor was slowly cooled to room temperature, and then the remaining gas was slowly released. The remaining reaction solution in the reaction kettle was transferred to a one-necked bottle, acidified to PH=1 by adding 1M hydrochloric acid, extracted with ethyl acetate, collected the organic phase, removed the solvent in vacuo, and separated through a silica gel column (eluent: petroleum ether / ethyl acetate =6 / 1) 158 mg of 2-methoxyphenylpropynoic acid was obtained, and the yield was 90%. 1 H NMR (400MHz, CDCl 3 ...
Embodiment 3
[0045] Embodiment 3: the synthesis of 4-chlorophenylpropynoic acid
[0046]
[0047] Accurately measure potassium carbonate (552mg, 4mmol, 400mol%), n-tetrabutylammonium bromide (644.6mg, 2mmol, 200mol%), refined dichloromethane (5.0mL), 4-chlorophenylacetylene in the glove box (136.5mg, 1mmol), sequentially added to a 25mL reaction kettle, and the glove box was filled with CO 2 (2MPa). The reactor was closed and placed in an oil bath at 60°C for 18 hours. After the reaction was finished, the reactor was slowly cooled to room temperature, and then the remaining gas was slowly released. The remaining reaction solution in the reaction kettle was transferred to a one-necked bottle, acidified to PH=1 by adding 1M hydrochloric acid, extracted with ethyl acetate, collected the organic phase, removed the solvent in vacuo, and separated through a silica gel column (eluent: petroleum ether / ethyl acetate =6 / 1) 145 mg of 4-chlorophenylpropynoic acid was obtained, and the yield was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com