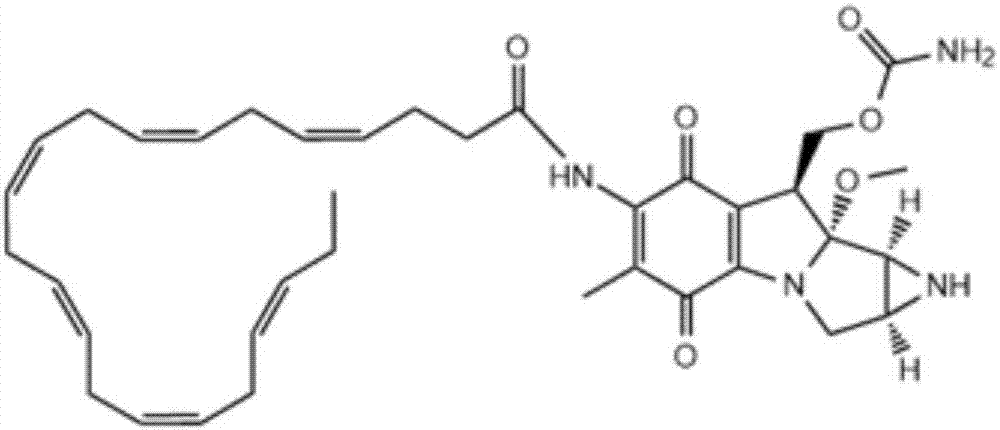
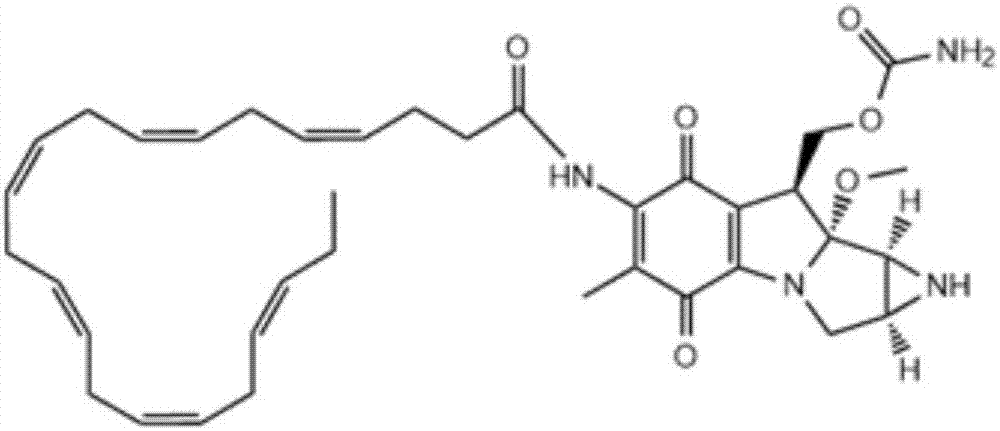
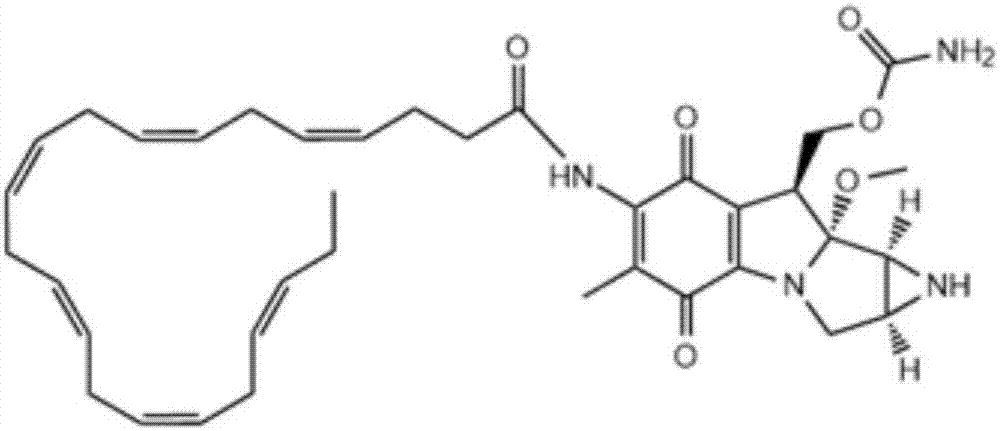
DHA (docosahexaenoic acid)-MMC (mitomycin C) derivative and preparation method and application thereof
A technology of mitomycin and derivatives, which is applied in the field of pharmacy, can solve side effects and other problems, and achieve the effects of reducing intraocular side effects, tumor suppression and enhanced targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Step 1, add 329mg (1mmol) of cis-4,7,10,13,16,19-docosahexaenoic acid and 173mg (1.5mmol) of N-hydroxysuccinimide to a 25ml round bottom bottle , 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride 574mg (3mmol) and 1.01g (10mmol) N-methylmorpholine, add dioxane 32.9g to dissolve after N 2 React at room temperature under protection for 48 hours, evaporate the solvent under reduced pressure, dissolve the residue in chloroform, wash with 30ml of 2N dilute hydrochloric acid, dry the chloroform layer and evaporate to dryness to obtain a yellow oil DHA-NHS, NHS 2 Store under protection.
[0054] Step 2, add the DHA-NHS 106.3mg (0.25mmol) that step 1 obtains in the 25ml round bottom bottle, 0.03mol / L HBS damping fluid (pH is 7.4) 10ml, add Mitomycin C 83.5mg (0.25 mmol), during which pH is monitored and 0.5mol / L aqueous sodium hydroxide solution is added as required to maintain pH 7.4; then the reaction solution is concentrated to dryness after stirring for 12 hour...
Embodiment 2
[0056] Step 1, add 329mg (1mmol) of cis-4,7,10,13,16,19-docosahexaenoic acid and 577mg (5mmol) of N-hydroxysuccinimide to a 25ml round bottom bottle, 957mg (5mmol) of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and 2.02g (20mmol) of N-methylmorpholine, after adding 2.3g of dioxane to dissolve , at N 2 React at room temperature for 4 hours under protection, evaporate the solvent under reduced pressure, dissolve the residue with dichloromethane, wash with 40ml of 1N phosphoric acid, dry the dichloromethane layer and evaporate to dryness to obtain a yellow oil DHA-NHS, NHS 2 Store under protection.
[0057] Step 2, add the DHA-NHS 106.3mg (0.25mmol) that step 1 obtains in the 25ml round bottom bottle, 0.01M borax solution buffer (pH is 9) 10ml, add Mitomycin C 83.5mg (0.25mmol) under stirring ), during which pH is monitored and 0.01mol / L potassium hydroxide aqueous solution is added as required to maintain pH 9; then the reaction solution is concentrated to dry...
Embodiment 3
[0059] Step 1, add 329mg (1mmol) of cis-4,7,10,13,16,19-docosahexaenoic acid and 115mg (1mmol) of N-hydroxysuccinimide to a 25ml round bottom bottle, 191mg (1mmol) of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and 101mg (1mmol) of N-methylmorpholine were added to dissolve in 15.0g of ether, and reacted for 96h. Evaporate the solvent under reduced pressure, dissolve the residue with carbon tetrachloride, wash with 20ml of 3N dilute phosphoric acid, dry the carbon tetrachloride layer and evaporate to dryness to obtain a yellow oil DHA-NHS, NHS 2 Store under protection.
[0060] Step 2, add DHA-NHS 106.3mg (0.25mmol) obtained in step 1 to a 25ml round bottom bottle, 0.025M potassium dihydrogen phosphate and disodium hydrogen phosphate mixed salt solution buffer (pH is 7) 10ml, add under stirring Mitomycin C 83.5mg (0.25mmol), during which pH was monitored and sodium carbonate powder was added as needed to maintain pH 7; then the reaction solution was stirred for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com