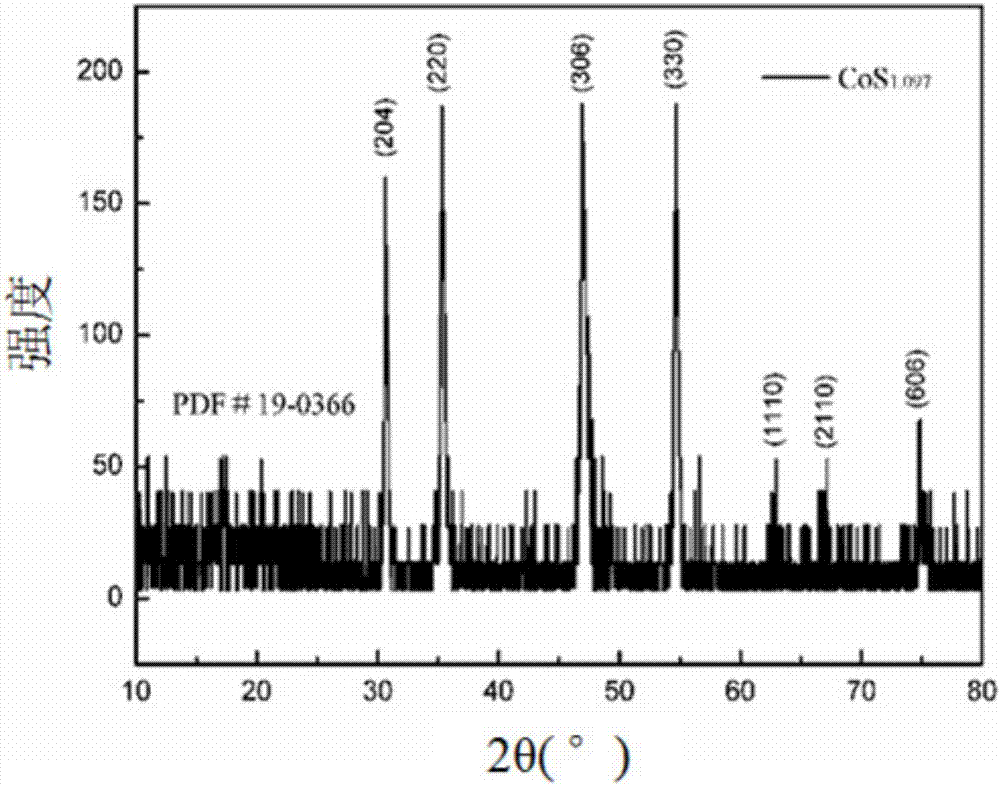
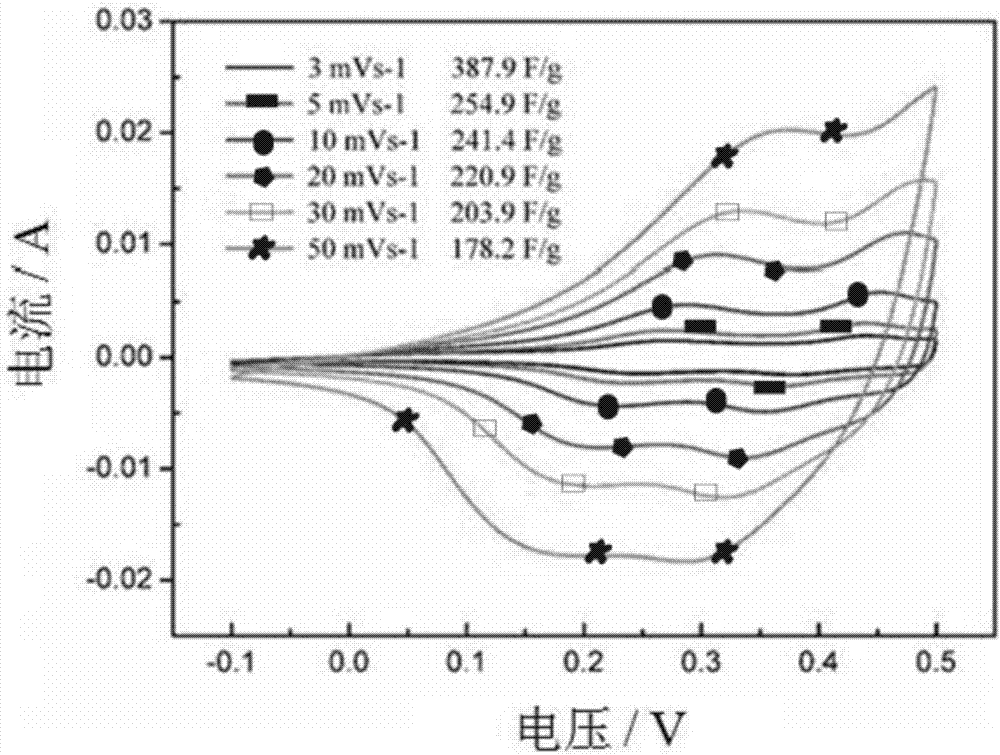
Recycling method for lithium cobaltate positive electrode material of lithium ion battery
A battery lithium cobalt oxide and cathode material technology, applied in battery recycling, recycling technology, waste collector recycling, etc., can solve the problems of high cost, cumbersome process, complicated process, etc., and achieve simple and easy effects and simple operation steps. , the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for recovering lithium cobalt oxide cathode material of lithium ion battery, comprising the steps of:
[0027] ①. Discharge the waste lithium cobalt oxide battery and disassemble it to obtain the positive electrode material of the battery;
[0028] ②. Ultrasonic immersion treatment of the above positive electrode material with N-methylpyrrolidone, and then filtering to obtain a bonded precipitate, and then sintering to obtain LiCoO 2 powder; wherein, the sintering temperature of the precipitate is 400°C, and the calcination time is 6h.
[0029] ③, the above LiCoO 2 The powder is reacted with a natural organic acid solution containing a hydrogen peroxide reducing agent to obtain a Li-containing + and Co 2+ Leaching solution; natural organic acid solution is ascorbic acid, malic acid or citric acid, its concentration is 0.4M / 100ml; LiCoO 2 The ratio of powder to natural organic acid solution is 20g / L.
[0030] ④. In the above leaching solution, the content o...
Embodiment 2
[0037] The recovery method of a lithium-ion battery lithium cobalt oxide cathode material in this embodiment is basically the same as in Example 1, and the effect achieved is also basically the same, the difference being:
[0038] The sintering temperature of the precipitate in step ② is 600°C, and the calcination time is 2h.
[0039] The natural organic acid solution in step ③ is ascorbic acid, malic acid or citric acid, and its concentration is 2M / 100ml.
[0040] LiCoO in step ③ 2 The ratio of the powder mass to the volume of the natural organic acid solution is 50g / L.
[0041] In step ④, the molar ratio of thiourea to the leachate is n(S):n(Co)=2.5 for mixed reaction.
[0042] The hydrothermal reaction in step ④ is carried out in a hydrothermal reaction kettle at a temperature of 180° C. and a reaction time of 18 hours.
[0043] In step ⑦, the filtrate is heated and concentrated at 98°C, and the filtrate is mixed with saturated Na 2 CO 3 The reaction time is 15min.
Embodiment 3
[0045] The recovery method of a lithium-ion battery lithium cobaltate positive electrode material in this embodiment is basically the same as in Example 1, and the effect achieved is also basically the same, the difference being:
[0046] The sintering temperature of the precipitate in step ② is 500°C, and the calcination time is 4h.
[0047] The natural organic acid solution in step ③ is ascorbic acid, malic acid or citric acid, and its concentration is 1.2M / 100ml.
[0048] LiCoO in step ③ 2 The ratio of the powder mass to the volume of the natural organic acid solution is 35g / L.
[0049] In step ④, the molar ratio of thiourea to the leachate is n(S):n(Co)=2 for mixed reaction.
[0050] The hydrothermal reaction in step ④ is carried out in a hydrothermal reactor at a temperature of 180° C. and a reaction time of 15 hours.
[0051] In step ⑦, the filtrate is heated and concentrated at 90°C, and the filtrate is mixed with saturated Na 2 CO 3 The reaction time is 45min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com