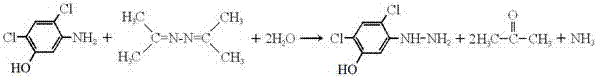
Synthetic process of 2,4-dichloro-5-hydroxyl phenyl hydrazine
A synthetic method, the technology of hydroxyphenylhydrazine, applied in the preparation of hydrazine, organic chemistry, etc., can solve the problems of low yield, large waste discharge, and high equipment requirements, and achieve easy control of the reaction, less waste discharge, and equipment undemanding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Put 178g of 2,4-dichloro-5-hydroxyaniline and 168g of acetone azine into a four-neck flask equipped with stirring, dropping funnel, thermometer and rectification column. The receiver of the rectification column is connected with a conduit for absorption Ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 54ml of water dropwise into the four-neck flask, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column, Acetone is produced at a column top temperature of 54°C to 58°C and ammonia gas is recovered. The water vapor and acetone azine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; End the reaction after no ammonia gas is released from the column receiver, distill all the water and acetonazine in the flask with negative pressure, wash the solid matter in the flask with absolute ethanol, and dry to obtain 2,4-dichloro-5-...
Embodiment 2
[0014] Put 178g of 2,4-dichloro-5-hydroxyaniline and 196g of acetonazine into a four-neck flask equipped with a stirring, dropping funnel, thermometer and rectification column, and a conduit is connected to the upper part of the rectification column for absorption Ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 63ml of water dropwise into the four-neck flask, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column, Acetone is produced at a column top temperature of 54°C to 58°C and ammonia gas is recovered. The water vapor and acetone azine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; End the reaction after no ammonia gas is released from the column receiver, distill all the water and acetonazine in the flask with negative pressure, wash the solid matter in the flask with absolute ethanol, and dry to obtain 2,4-dichlor...
Embodiment 3
[0016] Put 178g of 2,4-dichloro-5-hydroxyaniline and 224g of acetonazine into a four-neck flask with stirring, dropping funnel, thermometer and rectification column, and a conduit is connected to the upper part of the receiver of the rectification column for absorption Ammonia gas; start stirring, heat the solution to 120-130°C, slowly add 72ml of water dropwise into the four-neck flask, control the reaction temperature at 100°C-130°C, when the gas generated by the reaction enters the rectification column, Acetone is produced at a column top temperature of 54°C to 58°C and ammonia gas is recovered. The water vapor and acetone azine entering the rectification column are condensed and flow back to the flask to continue to participate in the reaction; End the reaction after no ammonia gas is released from the column receiver, distill all the water and acetonazine in the flask with negative pressure, wash the solid matter in the flask with absolute ethanol, and dry to obtain 2,4-di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com