Fluorescent conjugated polymer with side chain amidogen being protonized, preparing method, and application of fluorescent conjugated polymer
A technology of amino protonation and conjugated polymers, which is applied in the field of fluorescent sensing materials to achieve low detection limits and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of two monomers for the synthesis of conjugated polymers mainly includes the following main steps:
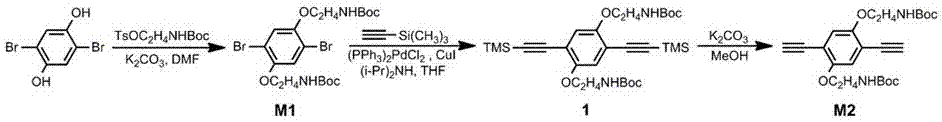
[0030] See attached figure 1 , the preparation of monomer 1 (M1): under argon protection, 2,5-dibromohydroquinone (0.80g, 3.00 mmol), 2-([tert-butoxycarbonyl]amino)ethyl 4-Methylbenzenesulfonate (2.13 g, 6.75 mmol), anhydrous potassium carbonate (4.15 g, 30.0 mmol), 15 mL N,N-dimethylformamide, heated to 70 ℃, refluxed and stirred for 24 h. Cool to room temperature, add 200 mL of deionized water, stir well, and filter to obtain a solid. After drying, the crude product is dissolved in a small amount of ethyl acetate, and washed with ethyl acetate / n-hexane (1:2) as eluent. The product was collected on a silica gel column and the solvent was removed, and the obtained product was recrystallized from ethyl acetate / n-hexane to obtain white flocculent crystals, which were dried. Yield 1.22 g (74%). 1 H NMR (400 MHz, CDCl 3 , δ): 1.46(s, 18H), 3.55(q, 4H), 4...
Embodiment 2
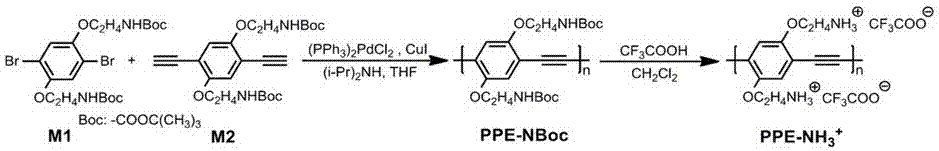
[0034] See attached figure 2 , which is the structure and synthetic route of the monomers required for the preparation of two fluorescent conjugated polymers; the synthesis of the conjugated polymers used for detection includes the following main steps:
[0035] See attached figure 2 , the preparation of the precursor PPE-NBoc: monomer M1 (0.277 g, 0.50 mmol), monomer M2 (0.222 g, 0.50 mmol), (PPh 3 ) 2 PdCl 2 (35 mg, 0.050 mmol), CuI (5 mg, 0.025 mmol) were added to a pre-dried and deoxygenated flask, and under argon gas flow, 8 mL of anhydrous tetrahydrofuran and 2 mL of anhydrous diisopropylamine were added. Under argon protection, reflux and stir at 60°C for 14 h. After the reaction, cool down to room temperature, filter the reaction solution with a 0.22 μm filter head, add dropwise to 200 mL of ice-cold diethyl ether to precipitate, centrifuge to obtain a solid, and finally dry it in a vacuum oven to obtain 0.212 g (51%) of an orange-red solid . 1 H NMR (400 MHz, CD...
Embodiment 3
[0038] With the conjugated polymer PPE-NH in embodiment 2 3 + Prepared solutions for the detection of aldehydes in aqueous environments.
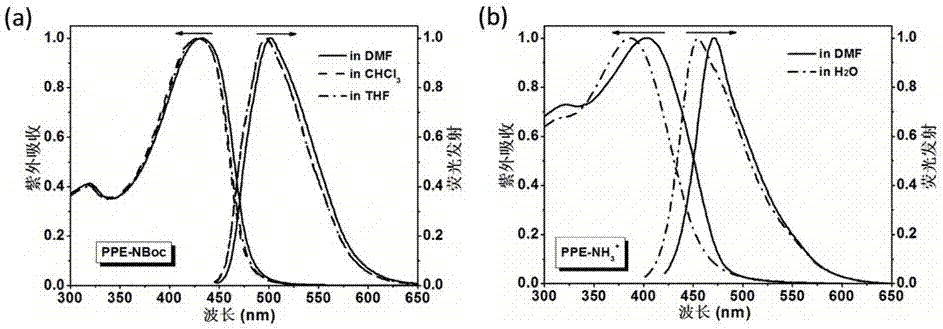
[0039] The concentration of the polymer solution is 2×10 -5 M (according to the repeating unit), first prepare two initial solutions of the analyte with different concentrations of 0,1 M and 0.5 M, take 2ml of polymer solution for each sample, add 20 μL of each analyte solution, and finally guarantee In the two groups of experiments, the effective concentrations of each analyte in the mixed solution were 1 mM and 5 mM respectively. After mixing, shake for 5 minutes, and then place it for 25 minutes before performing fluorescence tests one by one. All samples are prepared and used immediately. Repeat three times, the fluorescence intensity change at the maximum emission point in the fluorescence spectrum is the response. According to this method to obtain the data graph, see the appendix Figure 4 . The results show that the conjugated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com