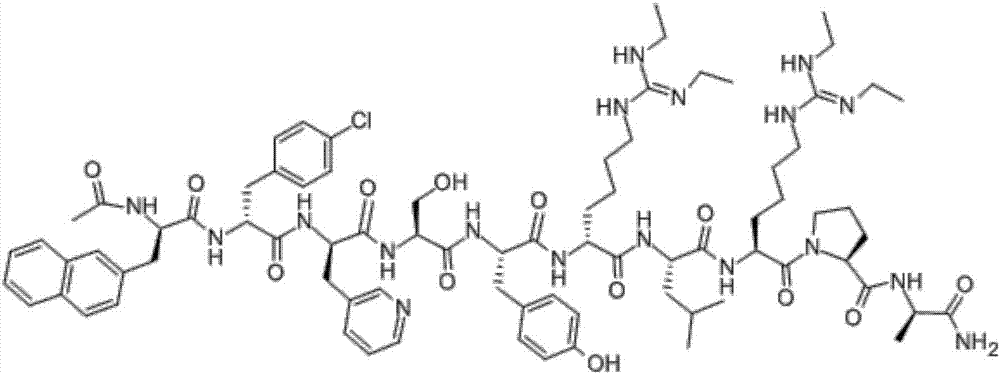
Segment method based solid-phase synthesis method of ganirelix acetate
A technique of Ganirelix and solid-phase synthesis, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as unfavorable production scale-up, long synthesis cycle, industrial scale-up, etc., and achieve industrial scale-up The effect of large-scale production, reducing the difficulty of synthesis, and reducing the cost of purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of Fmoc-D-Ala-Rink Amide Resin
[0056] Accurately weigh 20.0g of Rink Amide Resin (sub=0.65mmol / g) into the synthesis column, wash twice with 160mL DMF, add 160mL DCM to swell for 30min, filter off the DCM, wash twice with 160mL DMF; add 20% Deprotect 160ml of piperidine / DMF solution twice, react for 10min and 15min respectively; then wash twice with 160ml DMF, DCM, DMF respectively; filter off DMF, add Fmoc-D-Ala-OH / DIC / HOBT mixed DMF Solution [weigh 8.09g (26.00mmol) Fmoc-D-Ala-OH and 3.81g (28.20mmol) HOBT into a conical flask, add 80mL DMF solution and stir to dissolve, add 4.37ml (28.20 mmol) DIC, activated for 3min; reacted for 2h, took out the reaction solution, washed twice with 160mL DMF, added 250mL of end-capping reagent (50ml acetic anhydride, 42.5ml pyridine, 157.5mL DCM) for reaction for 2h, filtered off the reaction solution, respectively Wash 3 times with DMF, DCM, and methanol, and dry in vacuo to obtain 20.84 g of Fmoc-D-Ala...
Embodiment 2
[0057] Embodiment 2: Preparation of Fmoc-D-Ala-Rink Amide AM Resin
[0058] Accurately weigh 20.0g of Rink Amide AM Resin (sub=0.71mmol / g) into the synthesis column, wash twice with 200mL DMF, add 200mL DCM to swell for 30min, filter out the DCM, wash twice with 200mL DMF; 200ml of 20% piperidine / DMF solution was deprotected twice, reacted for 10min and 15min respectively; then washed twice with 200ml DMF, DCM, DMF respectively; DMF was filtered off, and Fmoc-D-Ala-OH / DIC / HOBT Mix DMF solution [weigh 8.84g (28.40mmol) Fmoc-D-Ala-OH and 4.22g (31.24mmol) HOBT into a conical flask, add 100mL DMF solution and stir to dissolve, add 4.84ml at low temperature (0°C) (31.24mmol) DIC, activated for 3min; reacted for 2h, took out the reaction solution, washed twice with 200mL DMF, added 300mL of capping reagent (60ml acetic anhydride, 51ml pyridine, 189mL DCM) to react for 2h, suction filtered off the reaction solution, respectively Wash 3 times with DMF, DCM, and methanol, and dry in ...
Embodiment 3
[0059] Embodiment 3: Preparation of Fmoc-Tyr(tBu)-CTC Resin
[0060] Weigh 80.0g (sub=1.00mmol / g) of CTC resin and place it in a synthesis column, wash twice with 500mL DMF, add 600mL DCM to swell for 30min; Tyr(tBu)-OH DCM solution 350ml, add 48.61ml (640.00mmol) DIPEA at low temperature (0°C), react for 60min, remove the reaction solution, add DCM / methanol / DIPEA (volume ratio 17:2:1) and mix 480ml of the solution was capped for 30min; then washed three times with DMF, DCM and methanol, and dried in vacuum to obtain 109.49g of Fmoc-Tyr(tBu)-CTC Resin; the degree of substitution was 0.63mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com