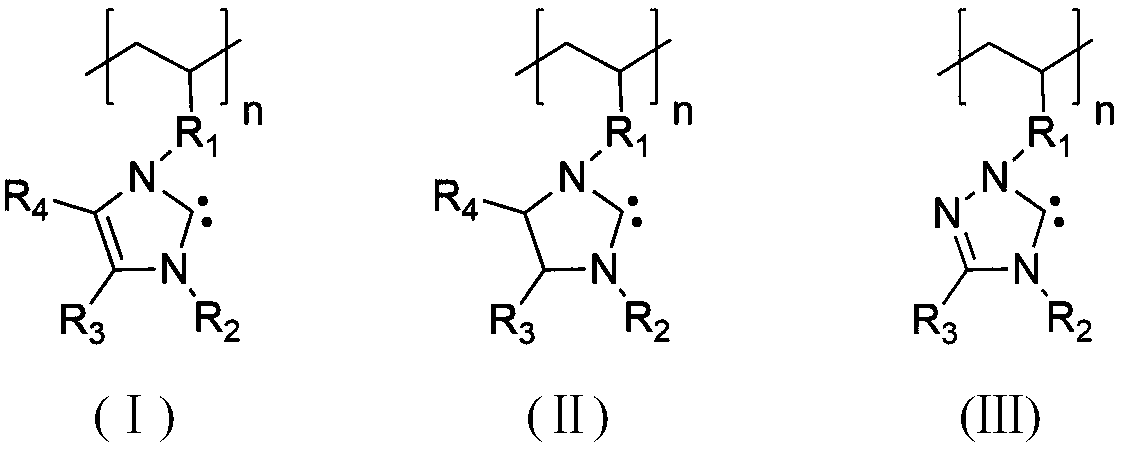
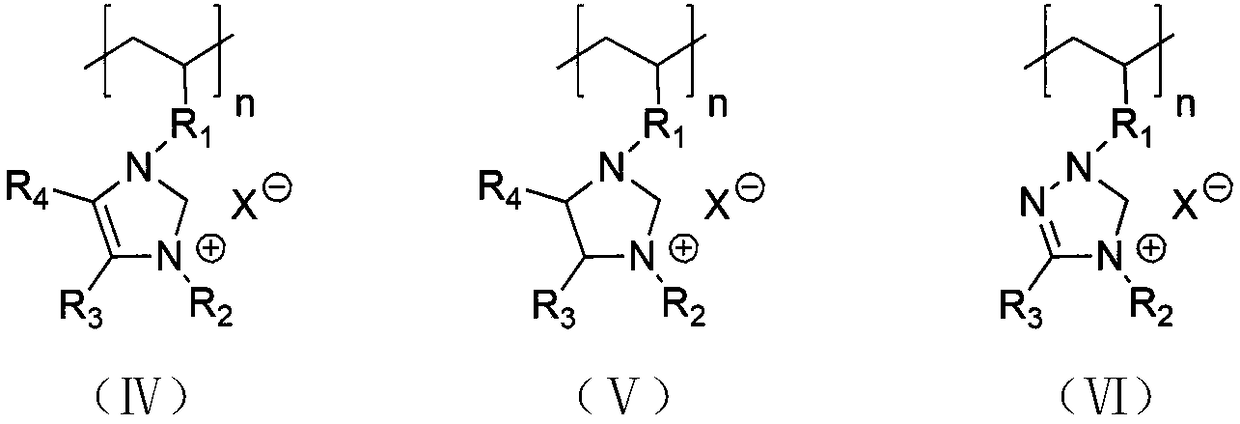
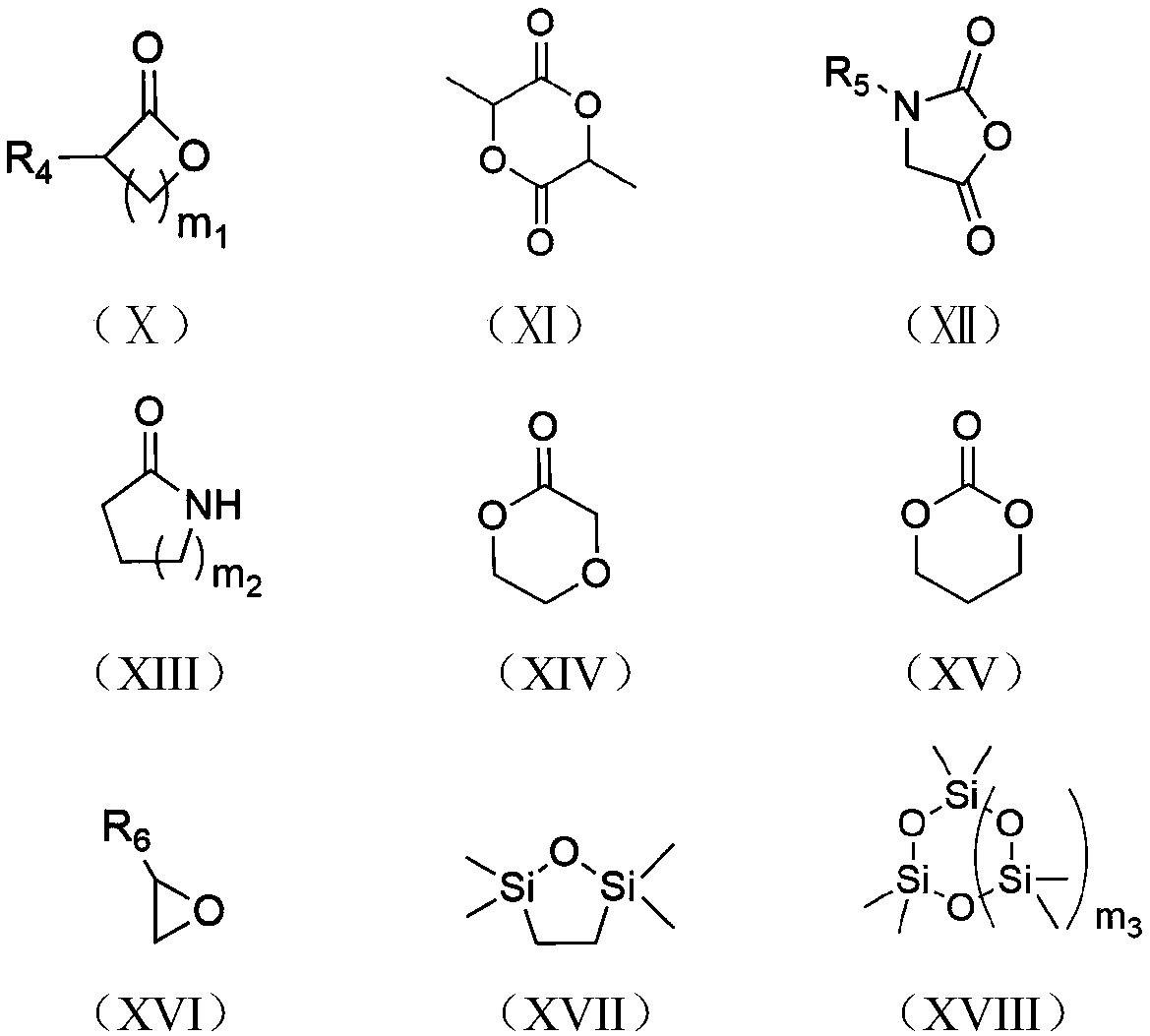Method for synthesizing brush-like polymers based on poly(n-heterocyclic carbene) structure self-catalyzed self-initiated ring-opening and the prepared brush-like polymers
A heterocyclic carbene and polymer technology, applied in the field of polymer preparation, can solve the problems of mild reaction conditions, limited polymerization degree of side chains, and low controllability, and achieve mild reaction conditions, controllable side chain structure, and expansion The effect of application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Under the protection of nitrogen, dissolve 0.1g of A in 5ml of tetrahydrofuran, then add 0.2ml of 1mol / L potassium tert-butoxide solution in tetrahydrofuran, stir at room temperature for 2h, then add 0.8g of caprolactone (the repeating unit of A and caprolactone The molar ratio was 1:30) at 0°C for 30 min, carbon dioxide was added to terminate the reaction, the reaction solution was poured into methanol, the precipitate was filtered and dried to constant weight to obtain 0.78 g of a reddish-brown solid with a conversion rate of 98%.
Embodiment 2
[0046] Under the protection of nitrogen, dissolve 0.1g of B in 10ml of tetrahydrofuran, then add 0.02ml of a tetrahydrofuran solution of 1mol / L potassium tert-butoxide, stir at 0°C for 10h, and then add 5.0g of caprolactone (the repeating unit of B and caprolactone The molar ratio of the ester is 1:200) at -80°C for 48 hours, the reaction solution was poured into methanol, the precipitate was filtered and dried to constant weight to obtain 3.8 g of reddish-brown solid with a conversion rate of 76%.
Embodiment 3
[0048] Under the protection of nitrogen, dissolve 0.1g of C in 20ml of dimethyl sulfoxide, then add 0.28ml of a tetrahydrofuran solution of 1mol / L potassium tert-butoxide, stir at room temperature for 10h, and then add 10.0g of caprolactone (the repeating unit of C React with caprolactone for 3 hours, pour the reaction solution into methanol, filter the precipitate and dry to constant weight to obtain 8.1 g of reddish-brown solid with a conversion rate of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com