Synthetic method for ultraviolet absorbent namely 4,4'-dihexyloxybenzophenone
A technology for the chemistry of dihexyloxybenzophenone and dihexyloxybenzophenone is applied in the field of synthesis of ultraviolet light absorbers to achieve the beneficial effects of less three wastes, suppression of side reactions, and environmental and producer labor protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
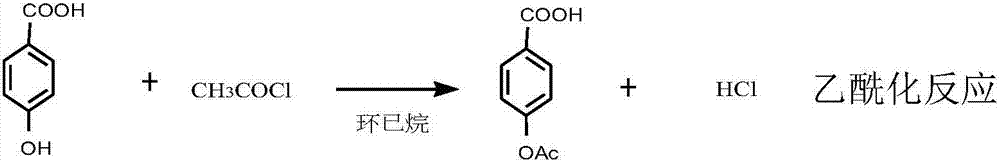
[0019] (1) Add 51.8g (0.375mol) of p-hydroxybenzoic acid to 100ml of cyclohexane, add dropwise 35.30g (0.45mol) of acetyl chloride at 30-35°C, and keep the reaction at 35-40°C for 2 hours after dropping. Atmospheric pressure distillation recovered excess acetyl chloride and solvent cyclohexane, and ethanol was added for recrystallization to obtain 65.5 g of p-acetoxybenzoic acid, with a yield of 97%;
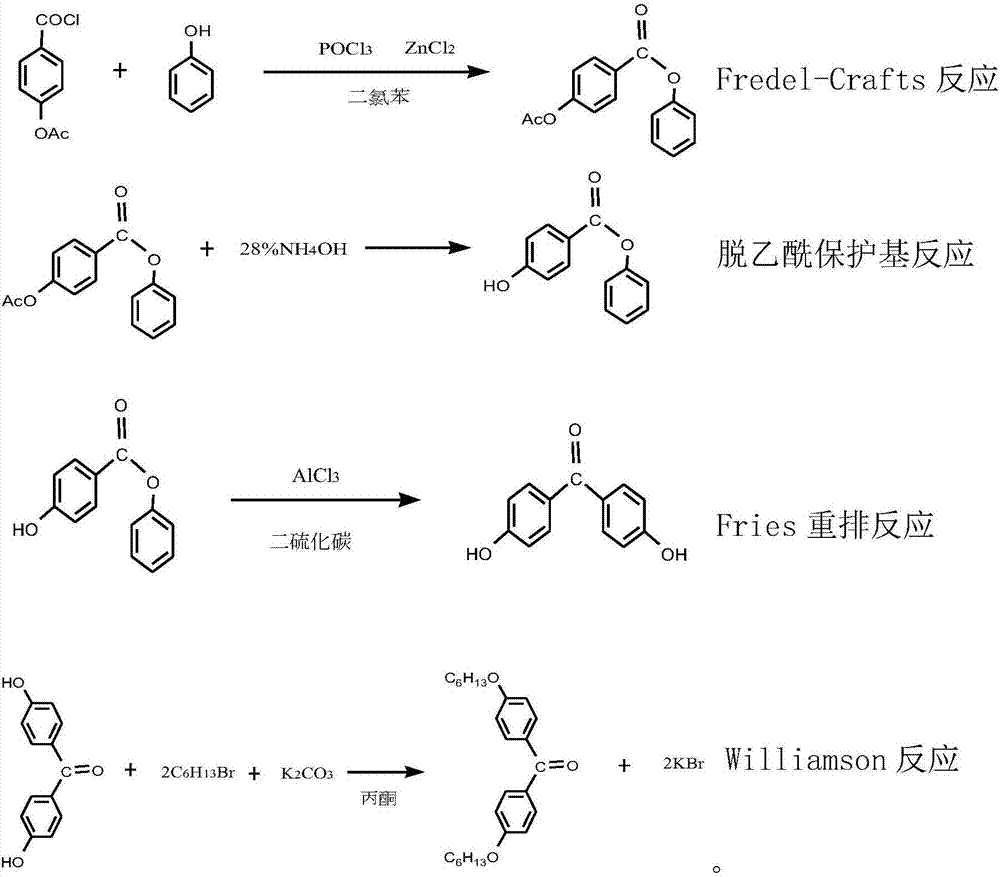
[0020] (2) Add 65.5g (0.36mol) of p-acetoxybenzoic acid, 35.8g (0.38mol) of phenol, phosphorus oxychloride 66 (0.43mol), and 98g (0.72mol) of zinc chloride into 180ml of dichlorobenzene , keep the reaction at 65-70°C for 2 hours, cool down to 30°C, pour it into 300ml of ice water in a trickle, let stand to remove the water, then wash with water until neutral; add 90ml of 28% ammonia water dropwise into the organic phase at 10-15°C , dripping and reacting at room temperature for 3 hours, standing to remove water, adding water and azeotropically distilling off the solvent dichloro...
Embodiment 2
[0024] (1) Add 51.8g (0.375mol) of p-hydroxybenzoic acid to 100ml of cyclohexane, add dropwise 36.10g (0.46mol) of acetyl chloride at 30-35°C, and keep the reaction at 35-40°C for 2 hours after dropping. Atmospheric distillation recovers excess acetyl chloride and solvent cyclohexane, and ethanol is added for recrystallization to obtain 65.4 g of p-acetoxybenzoic acid with a yield of 97%;
[0025] (2) Add 65.4g (0.36mol) of p-acetoxybenzoic acid, 37.7g (0.4mol) of phenol, 67.5g (0.44mol) of phosphorus oxychloride, and 98g (0.72mol) of zinc chloride into 180ml of dichlorobenzene , keep the reaction at 65-70°C for 2 hours, cool down to 30°C, pour it into 300ml of ice water in a trickle, let stand to remove the water, then wash with water until neutral; add 90ml of 28% ammonia water dropwise into the organic phase at 10-15°C After dripping and reacting at room temperature for 3 hours, let it stand to remove water, add water again and azeotropically distill off the solvent dichlor...
Embodiment 3
[0029] (1) Add 51.8g (0.375mol) of p-hydroxybenzoic acid to 100ml of cyclohexane, add dropwise 34.50g (0.44mol) of acetyl chloride at 30-35°C, and keep the reaction at 35-40°C for 2 hours after dropping. Atmospheric pressure distillation recovered excess acetyl chloride and solvent cyclohexane, and ethanol was added for recrystallization to obtain 65.5 g of p-acetoxybenzoic acid, with a yield of 97%;
[0030] (2) Add 65.5g (0.36mol) of p-acetoxybenzoic acid, 35.8g (0.39mol) of phenol, phosphorus oxychloride 66 (0.43mol), and 104g (0.73mol) of zinc chloride into 180ml of dichlorobenzene , keep the reaction at 65-70°C for 2 hours, cool down to 30°C, pour it into 300ml of ice water in a trickle, let it stand to remove the water, and then wash it until neutral; add 120ml of 28% ammonia water to the organic phase at 10-15°C , dripping and reacting at room temperature for 3 hours, standing to remove water, adding water and azeotropically distilling off the solvent dichlorobenzene, f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com