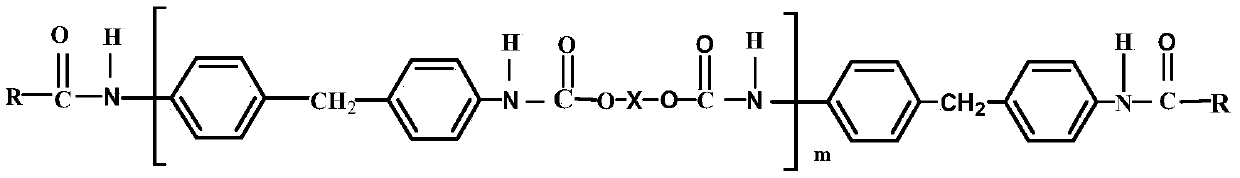Sulfonated hydroxypropyl chitosan modified biocompatible polyurethane and preparation method thereof
A technology of sulfonated hydroxypropyl chitosan and sulfonated hydroxypropyl shell is applied in the field of sulfonated hydroxypropyl chitosan modified biocompatible polyurethane and its preparation, and can solve the problem of improving blood compatibility. Obviously, the modified materials are expensive, the grafting rate is not high, etc., to achieve the effects of good anticoagulant performance and biocompatibility, improved hydrophilicity and anti-protein pollution performance, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
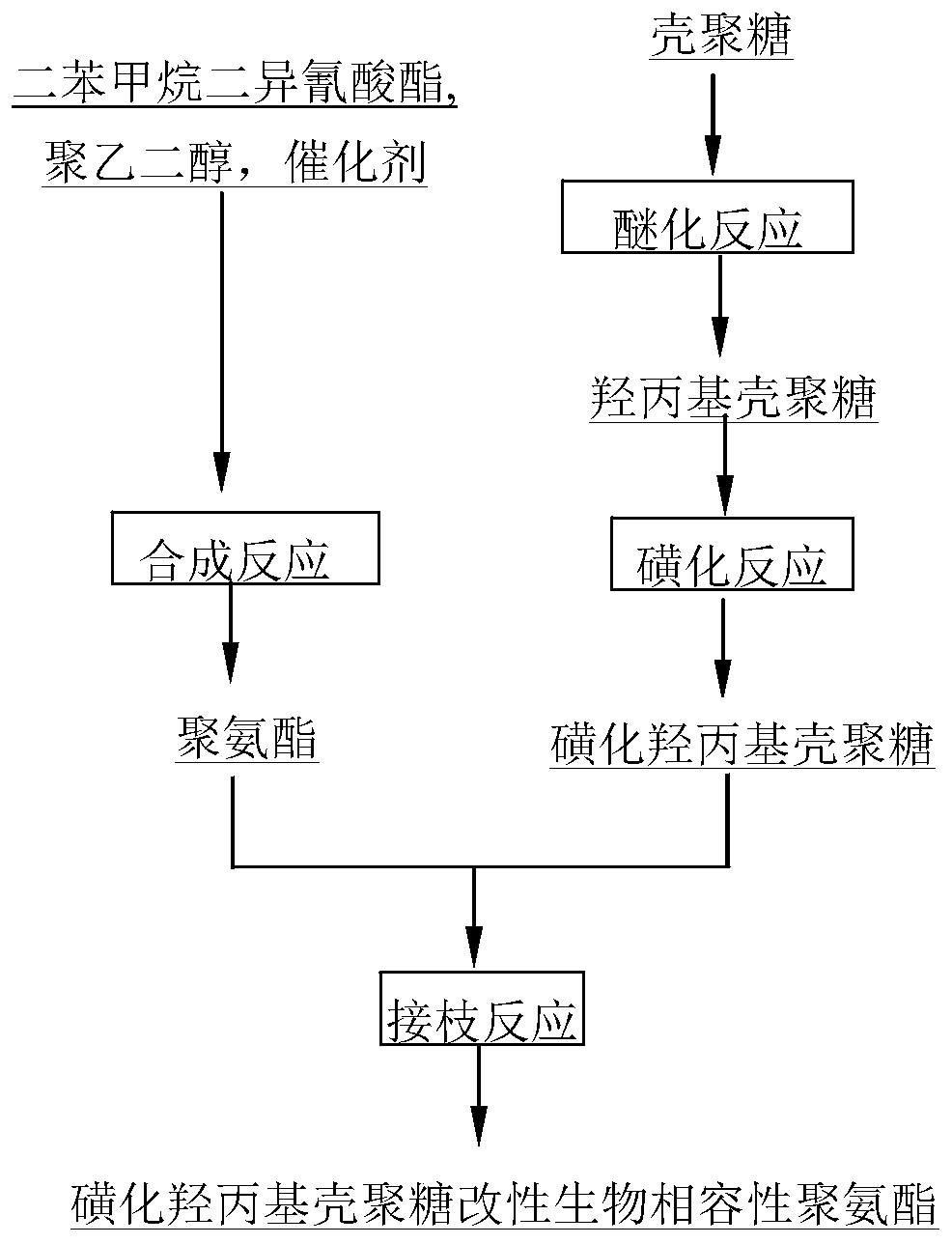
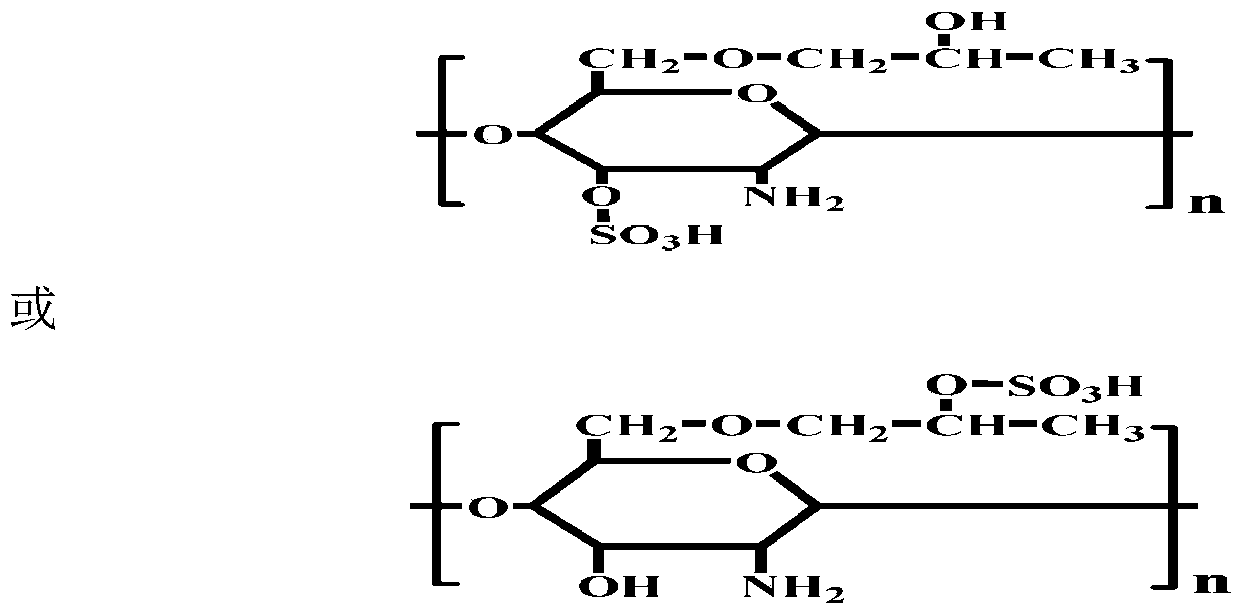
[0033]Take 5g CS as raw material, first use 33% NaOH solution to alkalinize, then add 50ml IPA and 50ml PO into the reactor, stir and react at 45°C for 5h, pour deionized water to dissolve and add acid Neutralization, followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. Mix 4ml of CSA and 20ml of formamide in an ice-salt bath to make a sulfonation reagent, then add 2g of HPCS, stir and react at 68°C for 3h, and undergo dialysis, deamination, redialysis, and concentration and drying to obtain SHPCS;
[0034] Using N,N-dimethylacetamide (DMAc) as a solvent, add diphenylmethane diisocyanate (MDI) and polyethylene glycol (PEG) in a molar ratio of 1.15:1 in sequence, and add octanoic acid sulfide after nitrogen gas for a period of time. Tin was mixed and stirred under the protection of anhydrous, oxygen and nitrogen, and reacted at 75°C for 2 hours to obtain a modified polyurethane prepolymer; then a certain amount of sulfonated hydroxypropyl ch...
Embodiment 2
[0036] Take 5g CS as raw material, first use 33% NaOH solution to alkalinize, then add 50ml IPA and 50ml PO into the reactor, stir and react at 45°C for 5h, pour deionized water to dissolve and add acid Neutralization, followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. Mix 4ml of CSA and 20ml of formamide in an ice-salt bath to make a sulfonation reagent, then add 2g of HPCS, stir and react at 68°C for 3h, and undergo dialysis, deamination, redialysis, and concentration and drying to obtain SHPCS;
[0037] Using N,N-dimethylacetamide (DMAc) as a solvent, add diphenylmethane diisocyanate (MDI) and polyethylene glycol (PEG) in a molar ratio of 1.15:1 in sequence, and add octanoic acid sulfide after nitrogen gas for a period of time. Tin was mixed and stirred under the protection of anhydrous, oxygen and nitrogen, and reacted at 75°C for 2 hours to obtain a modified polyurethane prepolymer; then a certain amount of sulfonated hydroxypropyl c...
Embodiment 3
[0039] Take 5g CS as raw material, first use 33% NaOH solution to alkalinize, then add 50ml IPA and 50ml PO into the reactor, stir and react at 45°C for 5h, pour deionized water to dissolve and add acid Neutralization, followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. Mix 4ml of CSA and 20ml of formamide in an ice-salt bath to make a sulfonation reagent, then add 2g of HPCS, stir and react at 68°C for 3h, and undergo dialysis, deamination, redialysis, and concentration and drying to obtain SHPCS;
[0040] Using N,N-dimethylacetamide (DMAc) as a solvent, add diphenylmethane diisocyanate (MDI) and polyethylene glycol (PEG) in a molar ratio of 1.15:1 in sequence, and add octanoic acid sulfide after nitrogen gas for a period of time. Tin was mixed and stirred under the protection of anhydrous, oxygen and nitrogen, and reacted at 75°C for 2 hours to obtain a modified polyurethane prepolymer; then a certain amount of sulfonated hydroxypropyl c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com