Method for over-expressing cecropin in cordyceps militaris
A technology of cecropin antibacterial peptide and Cordyceps militaris, applied in the field of transgenic, can solve the problems of reduced immunity of livestock and poultry, drug residues, threats to human health, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Construction of overexpression vector:
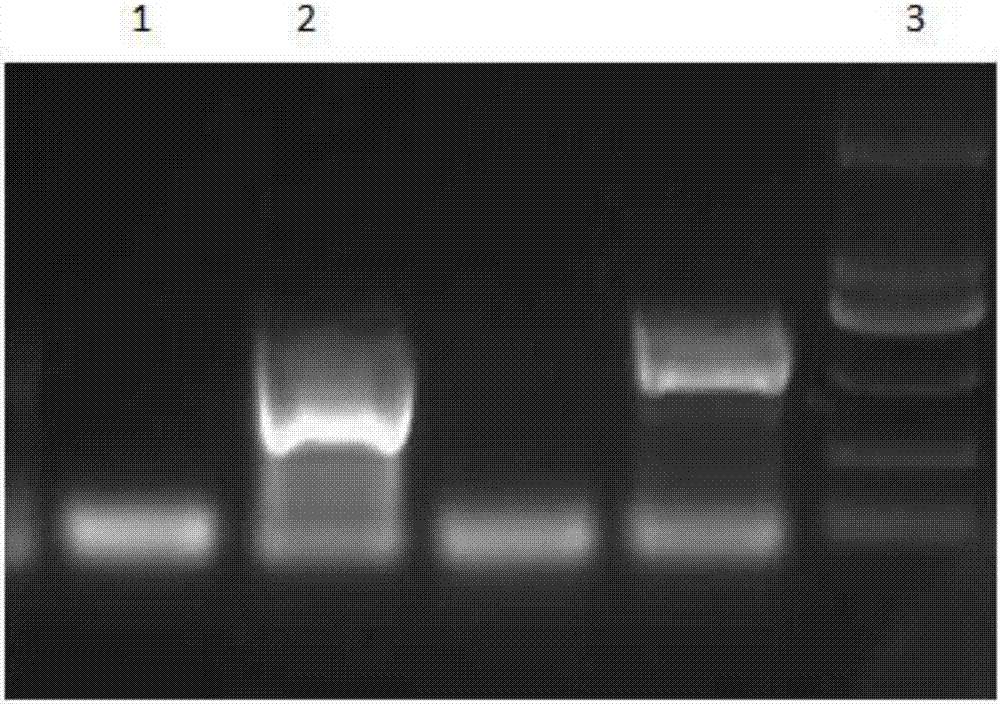
[0041] (1) Collect the mycelium of Cordyceps militaris on the PDA, extract DNA; use primer PR1 / PR2 to amplify the promoter fragment of HSP70 gene of Cordyceps militaris, use enzyme cutting site EcroR I / SmaI, digest, digest and connect PDHt-bar , obtaining the recombinant vector PDHt-HSP;
[0042] PR1: CGGAATTCCCCCTAAATCACAAGCTTGGTCCG;
[0043] PR2: TCCCCCGGGGTTGGCGGCTTCTGTGTGTGTA.
[0044] The PCR reaction system is:
[0045] Reagent reagent Volume (μL) PCR Magic Mix 2.0 25 Primer1 2 Primer2 2 Template DNA 3 wxya 2 o
18 Total 50
[0046] PCR reaction program: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s, annealing at 50°C for 30 s, extension at 72°C for 3 min, a total of 30 cycles; finally fill-up at 72°C for 10 min, and store at 10°C.
[0047] (2) Use the upstream primer Ple for TCCCCCGGGATGGGCTTCGGCTGCAACGG; the downstream primer Ple-BamH I-revCGGG...
Embodiment 2
[0049] Transformation in Homologous Recombination Vector Cordyceps militaris
[0050] (Agrobacterium tumefaciens-mediated genetic transformation of filamentous fungi)
[0051] 1) Select a freshly cultivated Agrobacterium containing PDHt-HSP-CAD vector from the LB plate (containing 50 μg / mL kanamycin)
[0052] AGL-1 single colonies were inoculated in 5 mL LB liquid medium (containing 50 μg / mL kanamycin), cultured overnight at 220 rpm at 28°C.
[0053] 2) Collect the bacterial cells by centrifugation the next day, resuspend with an equal volume of liquid induction medium IM, and pipette an appropriate amount of the resuspension to 5 mL of liquid induction medium IM to make OD660=0.14-0.2.
[0054] 3) Shake culture at 28°C and 220rpm for 4-6h, and the concentration of the bacterial solution should be between OD660=0.5-0.8.
[0055] 4) Cultivation and collection of Cordyceps militaris spores: In ultra-clean work, transfer the fresh hyphae activated on PDA medium (25°C, 20d) to a...
Embodiment 3
[0061] Bacteriostatic effect detection of transgenic strains
[0062] 1) Mycelia culture
[0063] Collect the fresh spore suspension of wild-type bacterial strain and transgenic bacterial strain of embodiment 2 respectively, concentration is adjusted to 10 8 cells / mL, pipette 300 μL, inoculate into 100 mL of SDB medium, culture at 25°C, 150rmp shaker for 3 days;
[0064] 2) Mycelia were collected by filtration, freeze-dried, and stored at -80°C for later use;
[0065] 3) The mycelium was homogenized in PBS buffer solution, and the homogenate was broken, centrifuged at 4°C and 8000rmp for 5min, and the supernatant was collected;
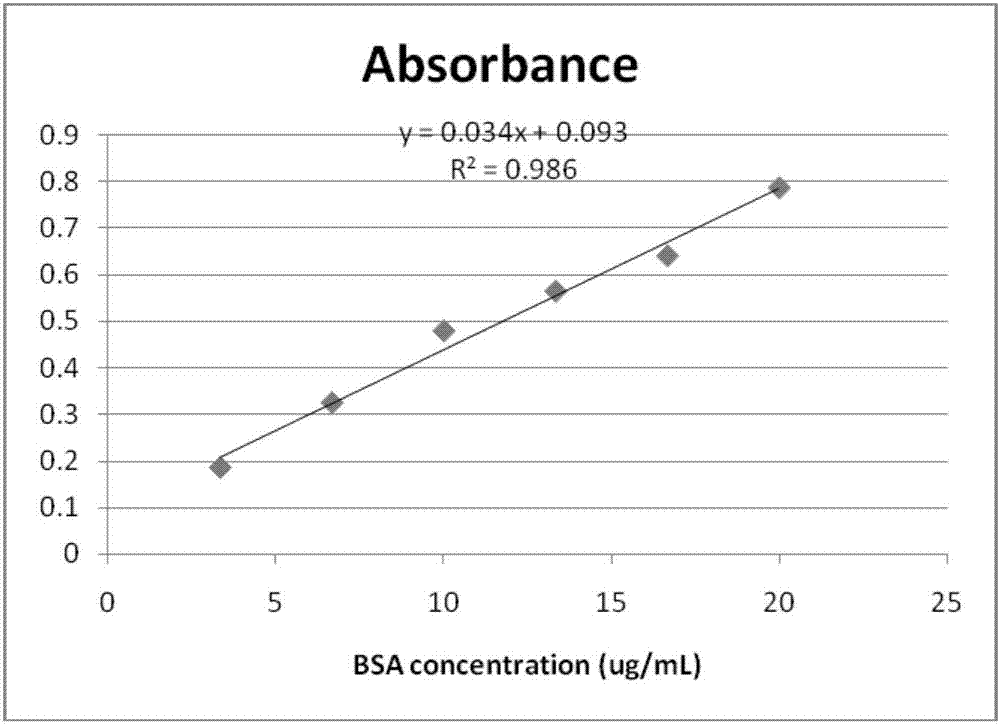
[0066] 4) Protein quantification was performed by Bradford method.
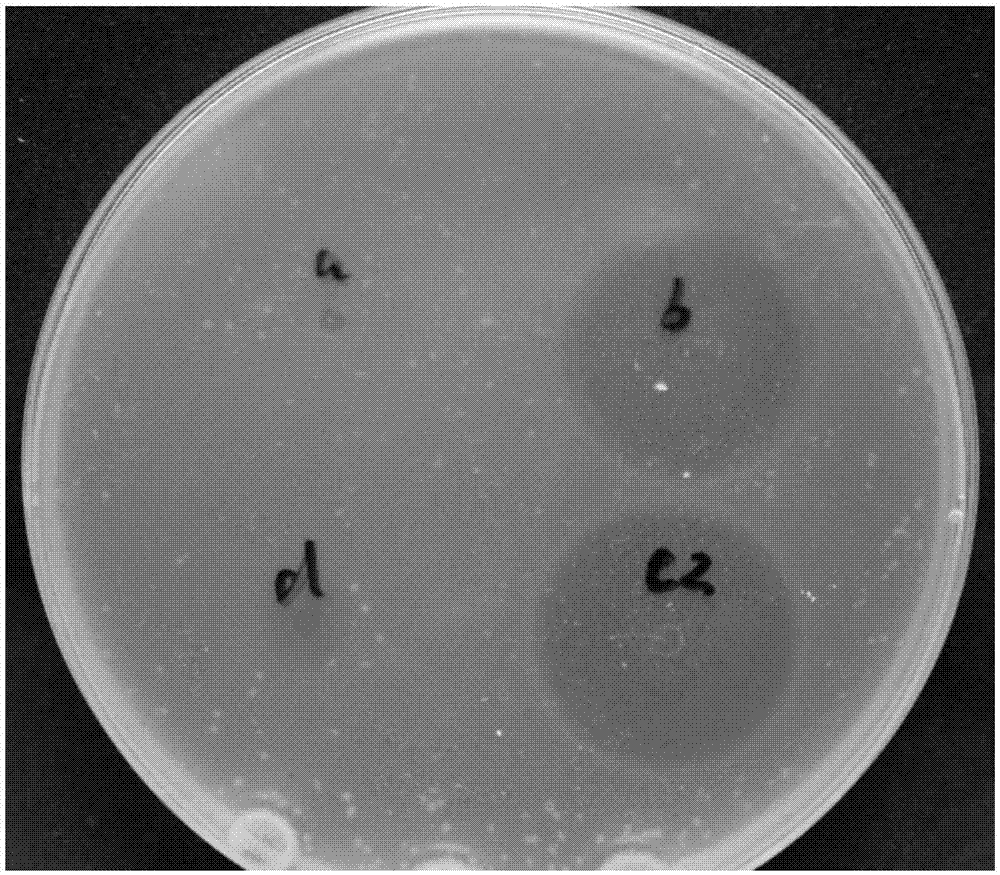
[0067] Bacteriostasis test: E. coli DH5a stored at -80°C was inoculated into 5 mL of LB medium with an inoculation loop, and cultured overnight at 37°C and 250 rpm on a shaker for 10-12 hours. The Escherichia coli cultured overnight was inoculated into fresh LB medium at a ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com