Gene mining and application method of phospholipase D
A phospholipase and coding gene technology, which is applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of long fermentation time, affecting the efficiency of phosphatidylserine, and insufficient understanding of enzyme properties, and achieve short fermentation cycle, high-efficiency expression, The effect of large application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This example illustrates the method for screening phospholipase D based on database gene mining technology.
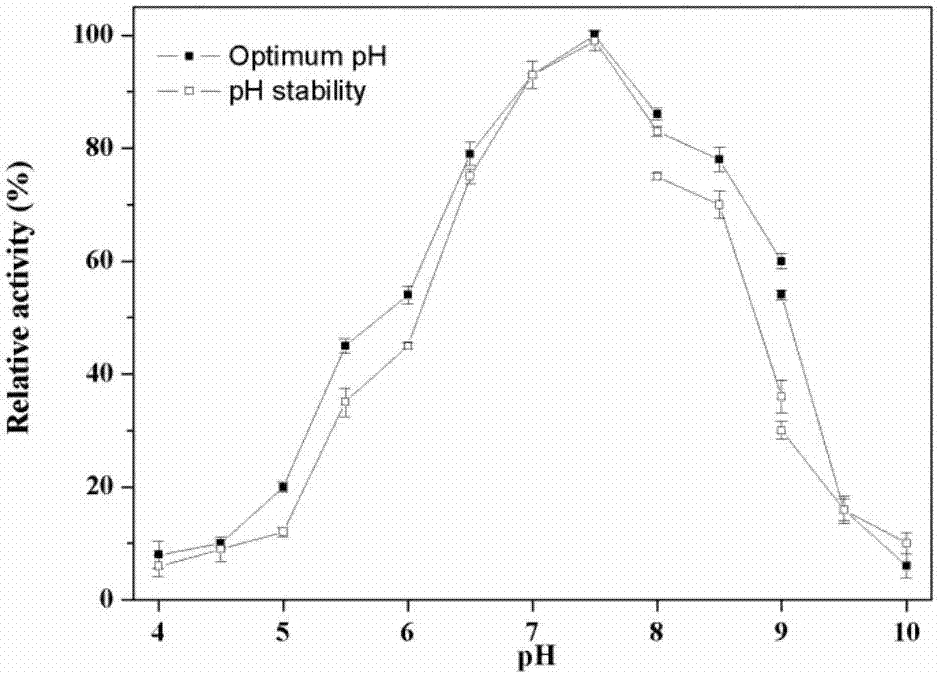
[0045] According to the literature on the exogenous expression of phospholipase D, the amino acid sequence of phospholipase D with high phosphatidyltransfer activity verified by experiments was selected as a template, and BLAST was performed in the NCBI database, and the screening was carried out according to the set screening criteria. A series of phospholipase D amino acid sequences are obtained, and then the amino acid sequence of the target phospholipase D is obtained by constructing a phylogenetic tree analysis and screening, thereby obtaining its coding gene sequence.
Embodiment 2
[0047] This example illustrates the cloning method of the gene encoding phospholipase D.
[0048] With the synthetic nucleotide sequence design primer (P1, P2), the coding gene of phospholipase D is amplified by PCR:
[0049] Primer P1: 5'-CG GGA TCC ATG CTG CGT CGC CTG CAT
[0050] Primer P2: 5'-CCC AAG CTT TTA CAG ACT ACA CAC ACC
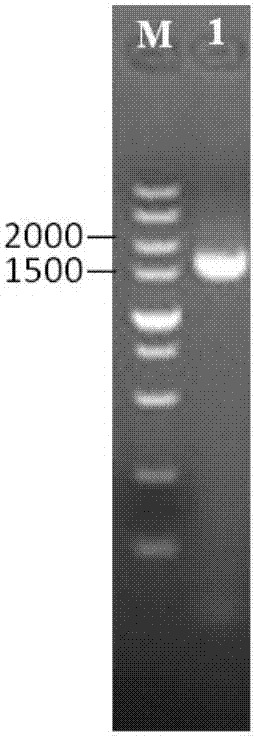
[0051] The PCR amplification reaction was carried out in a 50 μL system, and 25 μL Ex Taq (Premix), 20 μL ddH 2 O, 2 μL template DNA, 1.5 μL each for upstream and downstream primers. The reaction conditions were 30 cycles of denaturation at 94°C for 3 minutes: denaturation at 94°C for 30 s, annealing at 57°C for 30 s, extension at 72°C for 1 min, and a total of 30 cycles; final extension at 72°C for 10 min. The PCR products were identified by nucleic acid electrophoresis, recovered and purified by tapping the gel, and the recovered products were cloned into the pMD19-T vector to construct a recombinant cloning plasmid, which was transforme...
Embodiment 3
[0053] This example takes pET-28a(+) as an example to illustrate the construction process of the recombinant expression plasmid.
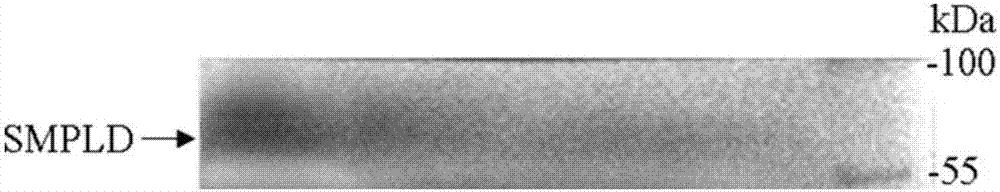
[0054] pET-28a(+) has a T7 promoter and 6×His-tag tag, and the pET-28a(+) plasmid and the verified recombinant cloned plasmid containing the target gene are double digested with BamH I and Hind III at the same time, After digestion at 37°C for 3 hours, carry out nucleic acid electrophoresis verification and tap gel to recover the target gene and target plasmid. The recovered product was ligated with T4 DNA ligase at 16°C overnight, and the ligated product was transformed into E.coli JM109 competent cells and spread on the Cultivate in LB solid culture of mycin resistance (50mg / mL) for 8-10h, pick multiple positive transformants and culture them in LB liquid medium containing kanamycin (50mg / mL), at 37°C, 220rpm The plasmid was extracted and named pET-28a(+)-pld after culturing under the condition for 10-12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com