Method for synthesizing carbamate by using olefin, amine, carbon dioxide and Togni reagent
A carbamate, carbon dioxide technology, applied in the fields of medicine, chemical industry, and organic synthesis, can solve problems such as threats to human life and health, industrial application restrictions, environmental pollution, etc., achieves good application prospects, is beneficial to industrial production, and is compatible with Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
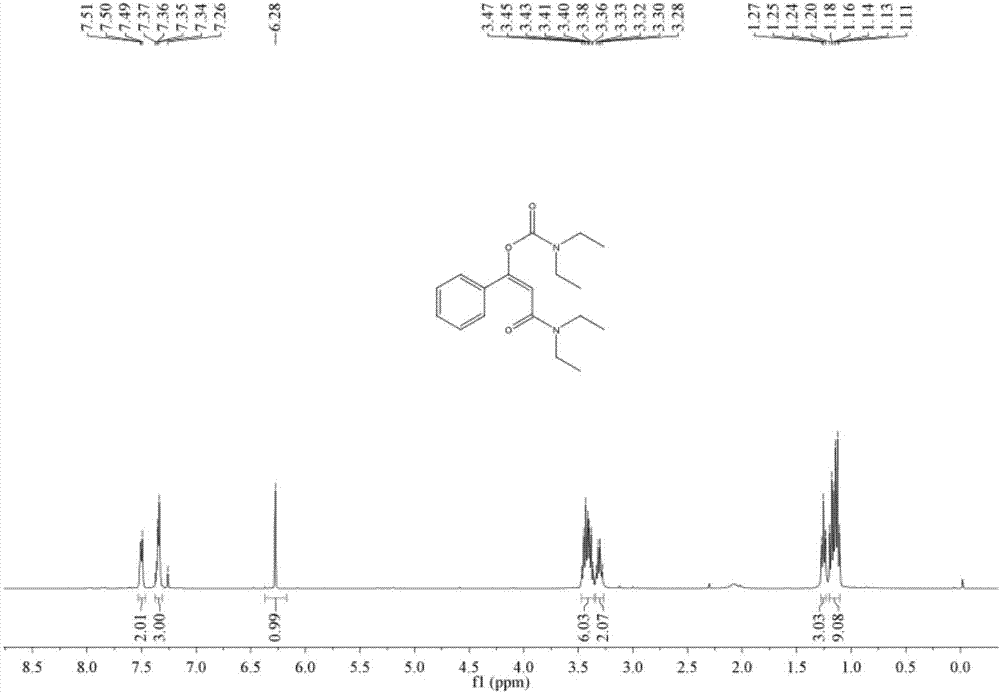
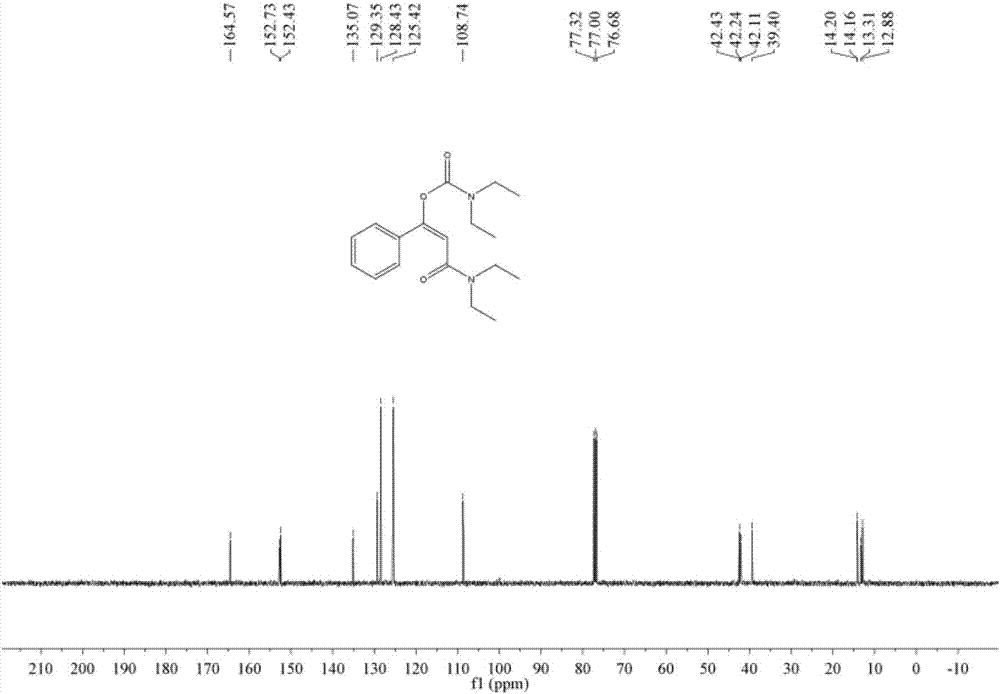
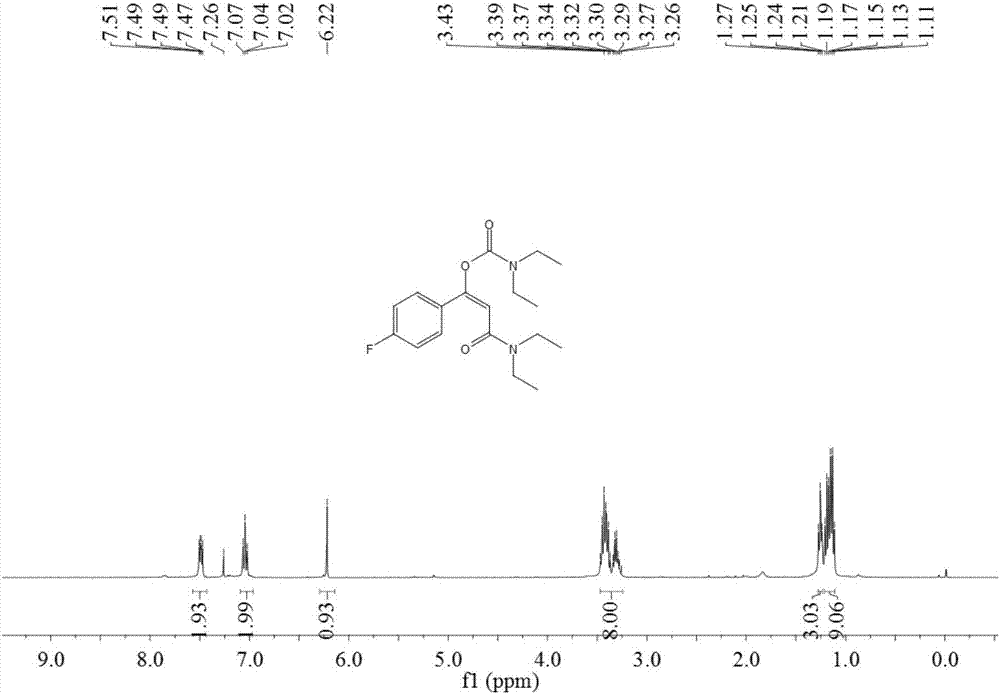
[0049] Add 1.5 ml of dimethyl sulfoxide, 0.20 mmol of styrene, 1.0 mmol of diethylamine, and 0.24 mmol of 1-trifluoromethyl-1,2-phenyliodyl-3(1H) to the autoclave successively. - Ketone, add 0.02 mmol copper acetate, then fill with 4MPa carbon dioxide, stir and react at 60°C for 8 hours, stop heating and stirring, cool to room temperature, slowly release carbon dioxide to normal pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was a mixed solvent of petroleum ether:ethyl acetate with a volume ratio of 2:1, and the yield was 90%.
Embodiment 2
[0051] Add 1.5 ml of dimethyl sulfoxide, 0.20 mmol of styrene, 1.0 mmol of diethylamine, and 0.24 mmol of 1-trifluoromethyl-1,2-phenyliodyl-3(1H) to the autoclave successively. -ketone, then add 0.02 mmol cuprous iodide, then fill with 4MPa carbon dioxide, stir and react at 60°C for 8 hours, stop heating and stirring, cool to room temperature, slowly release carbon dioxide to normal pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 51%.
Embodiment 3
[0053] Add 1.5 ml of dimethyl sulfoxide, 0.20 mmol of styrene, 1.0 mmol of diethylamine, and 0.24 mmol of 1-trifluoromethyl-1,2-phenyliodyl-3(1H) to the autoclave successively. - Ketone, then add 0.02 mmol copper trifluoromethanesulfonate, then fill with 4MPa carbon dioxide, stir and react at 60°C for 8 hours, stop heating and stirring, cool to room temperature, slowly release carbon dioxide to normal pressure. The reaction solution was washed with 10 mL of water, extracted three times with ethyl acetate (15 mL each time), the organic phases were combined and dried over anhydrous magnesium sulfate, concentrated by distillation under reduced pressure to obtain a crude product, and then separated and purified by column chromatography to obtain the target product. The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 2:1, and the yield was 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com