Fluorescent compound with aggregation-induced luminescence properties and application thereof to cell fluorescence imaging field
A technology for aggregation-induced luminescence and fluorescent compounds, which is applied in the preparation of organic compounds, fluorescence/phosphorescence, carbon-based compounds, etc., can solve problems such as constraints and the influence of fluorescent molecules, and achieve easy operation, sensitive and fast cell staining, and light resistance good bleaching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of C3NDPA molecule: Weigh 0.2g compound 1, 0.142g compound 2, 0.015g palladium acetate, 0.5g cesium carbonate, 0.04g tert-butylphosphine, add 20mL toluene, reflux reaction under nitrogen protection for 24 hours, after cooling, use 200-300 mesh silica gel powder was separated by column chromatography using dichloromethane as a developer to obtain C3NDPA as a red solid powder with a yield of 0.168 g and a yield of 65%.
[0043]
[0044] 1 H-NMR (500MHz, CDCl 3 )δ9.09(d, J=8.0Hz, 1H), 8.96(dd, J=4.6, 1.9Hz, 1H), 8.73(dd, J=7.9, 1.9Hz, 1H), 8.69(d, J=8.4 Hz,1H),8.18(dd,J=8.5,1.1Hz,1H),7.54(t,J=8.0,1H),7.50(dd,J=7.9,4.6Hz,1H),7.45(d,J= 8.1Hz,1H),7.30-7.23(m,4H),7.12-7.01(m,6H).
[0045] 13 C-NMR (125MHz, CDCl 3 )δ182.71, 153.43, 153.32, 152.27, 148.70, 135.64, 131.14, 130.58, 129.53, 129.00, 128.92, 127.57, 126.51, 126.47, 125.48, 124.72, 123.81, 123.5
[0046] HRMS (TOF LD + ): m / z=398.1429 (C 28 h 18 N 2 o + ,calcd=398.1414).
Embodiment 2
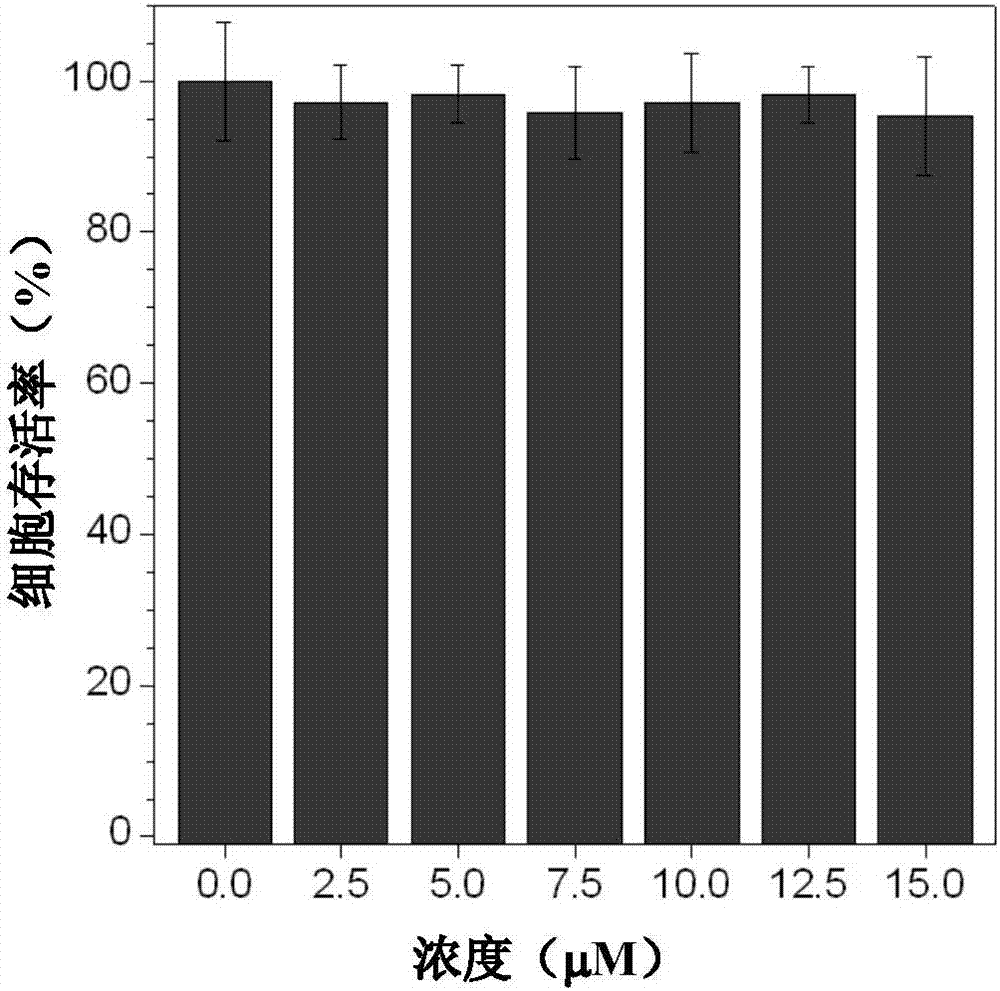
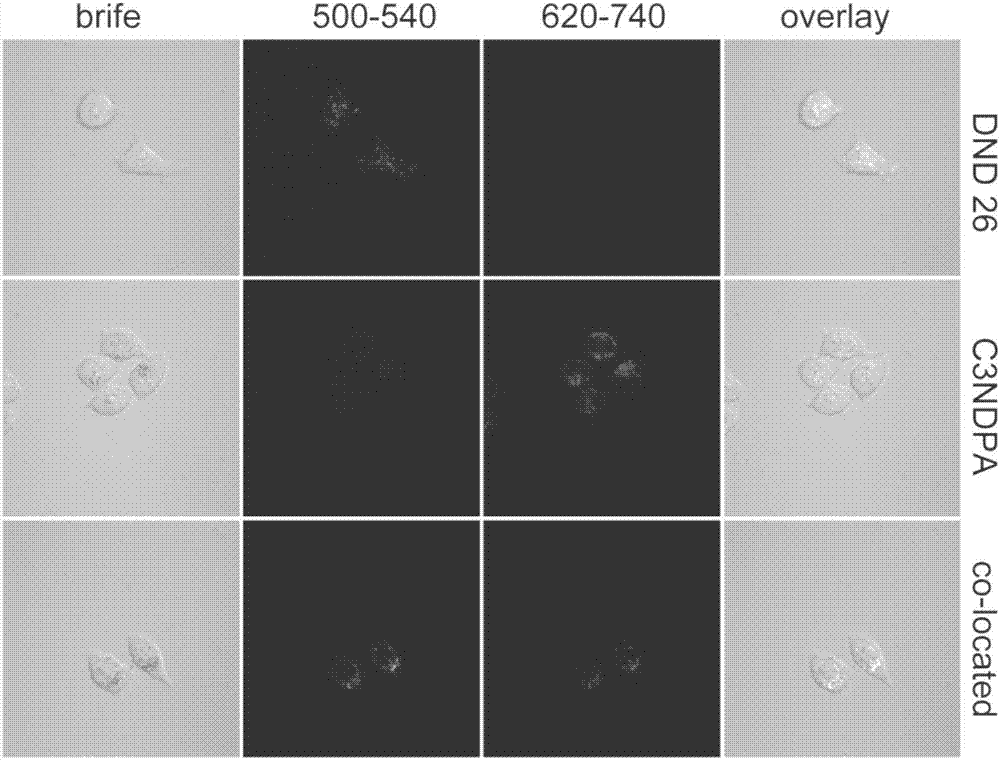
[0048] Dissolve the C3NDPA fluorescent dye in DMSO to obtain a probe solution (0.001mol / L), filter and sterilize it with a 0.22μm filter, add it to DMEM / F12 medium containing 15% FBS and 1% double antibody, shake Evenly, the working solution for cell staining was obtained. After co-culturing the working solution with adherent cells for a certain period of time, the staining was observed under a laser confocal microscope. figure 1 It showed that the cytotoxicity of the dye was very small, and the survival rate of Hela cells was above 95% after 48 hours of culture within 15 μmol / L concentration. Proved by dyeing experiments (for experimental results, see figure 2 ), the dye can quickly enter the living Hela cells within 1 h, and is mainly enriched in the cell lysosomes.
Embodiment 3
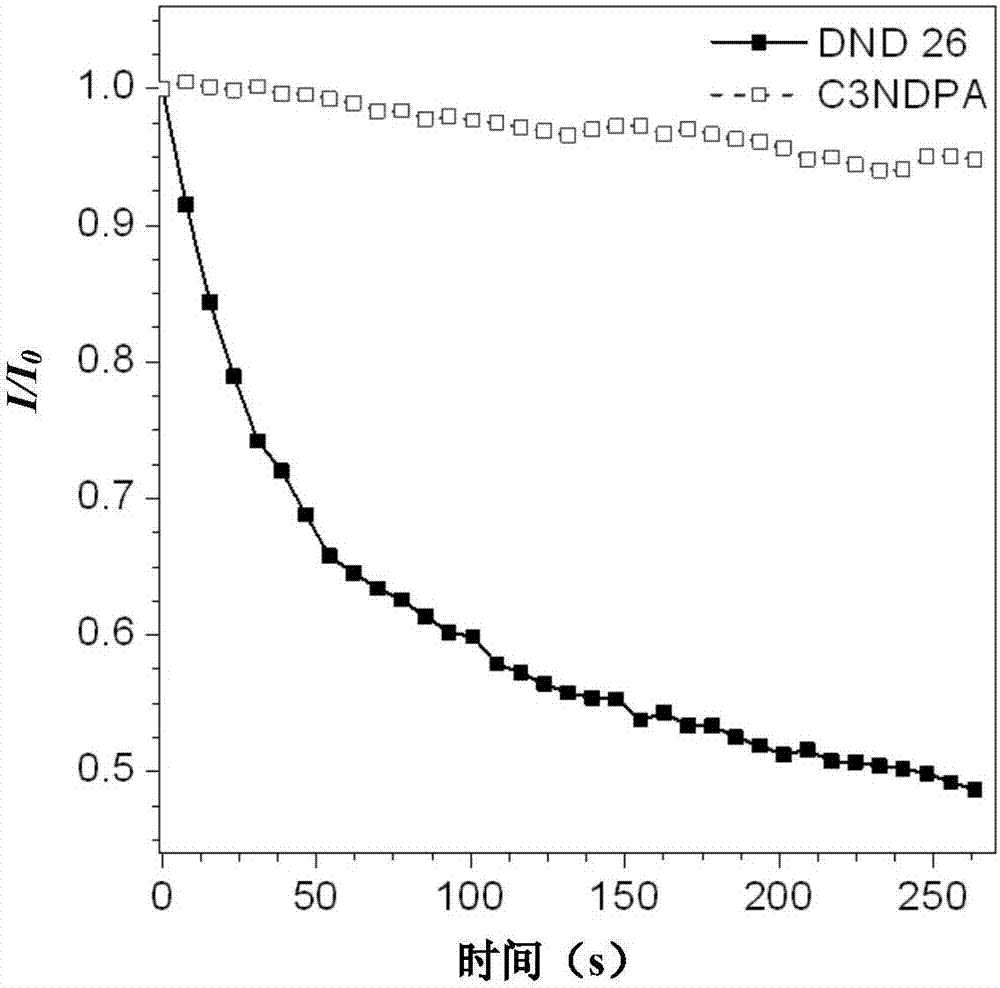
[0050] Dissolve the C3NDPA fluorescent dye in DMSO to obtain a probe solution (0.001mol / L), filter and sterilize it with a 0.22μm filter, add it to DMEM / F12 medium containing 15% FBS and 1% double antibody, shake Evenly, the working solution for cell staining was obtained. With this working solution and commercial dyes The adherent cells were co-stained under the same concentration of DND 26. After culturing for a certain period of time, laser scanning was performed under a laser confocal microscope to test the fluorescence signal intensity emitted by the two dyes. The results are shown in image 3 . image 3 The results show that the dye has excellent anti-photobleaching properties: comparable to commercial dyes Compared with DND 26, the signal attenuation of this dye is less than 5% after 250 s excitation light irradiation, while The DND26 dye signal decays by more than 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com