A kind of amylase mutant preparation method and its application
An amylase and mutant technology, applied in the fields of genetic engineering and genetic engineering, can solve the problems of large blindness, low frequency of beneficial mutations, large workload of artificial mutagenesis, etc., and achieves high specific activity, broad application prospects, and shortened transformation. effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Cloning of embodiment 1 amylase mutant encoding gene E87K
[0047] Using the recombinant plasmid pPIC9-amy6 of the gene amy6 cloned from T. leycettanus JCM12802 as a template, wherein the amino acid sequence encoded by amy6 is shown in SEQ ID NO: 1, primers were designed according to the instructions of the Fast Mutagenesis System, and then amplified.
[0048] (7) Table 1. Specific primers used for amylase mutant E87K
[0049]
Embodiment 2
[0050] The preparation of embodiment 2 amylase mutants
[0051] The recombinant plasmid pPIC9-amy6 was amplified using the Fast Mutagenesis System of Beijing Quanshijin Company to obtain the amylase mutant plasmid pPIC9-E87K and transformed into Pichia pastoris GS115 to obtain the recombinant yeast strain GS115 / E87K.
[0052] Take the GS115 strain containing the recombinant plasmid, inoculate it in a 1L Erlenmeyer flask with 300mL of BMGY medium, and culture it on a shaker at 30°C and 220rpm for 48h; then centrifuge the culture solution at 3000g for 5min, discard the supernatant, and use 100mL of 0.5% methanol for precipitation. The BMMY medium was resuspended, and placed again at 30°C, 220rpm to induce culture. Add 0.5 mL of methanol every 12 hours to keep the concentration of methanol in the bacterial solution at 0.5%, and take the supernatant for enzyme activity detection.
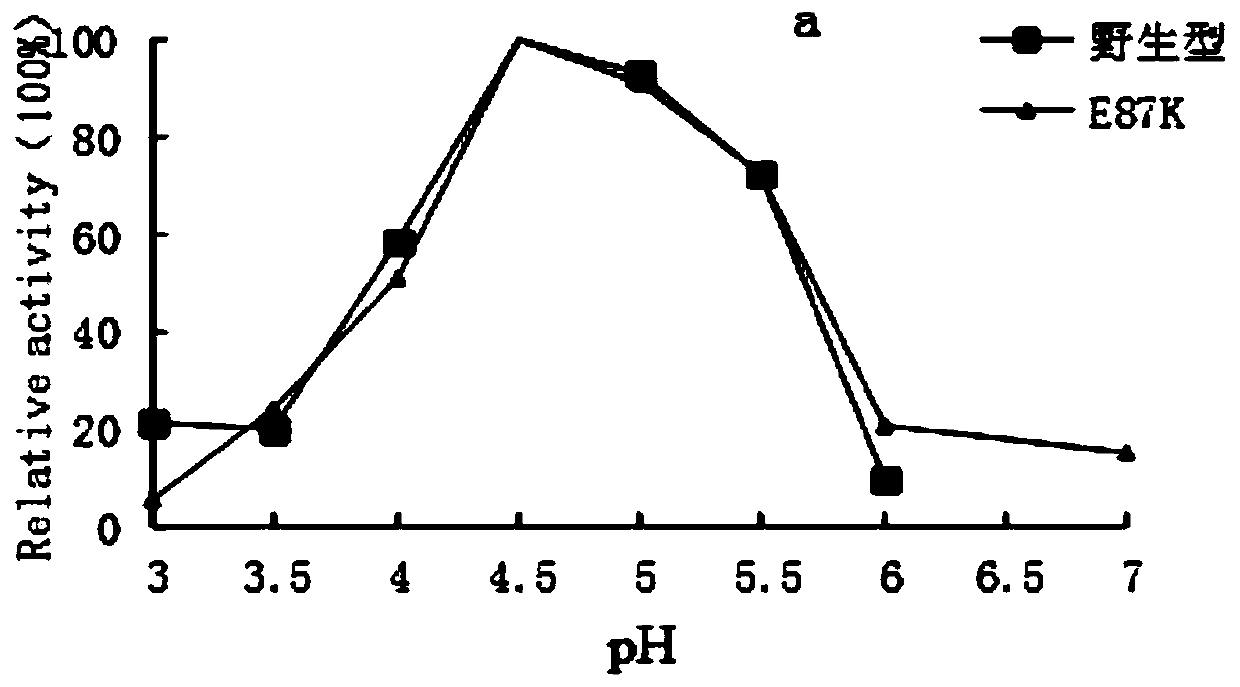
[0053] The optimum pH of the recombinant amylase mutant was 4.5, which was consistent with that of ...
Embodiment 3
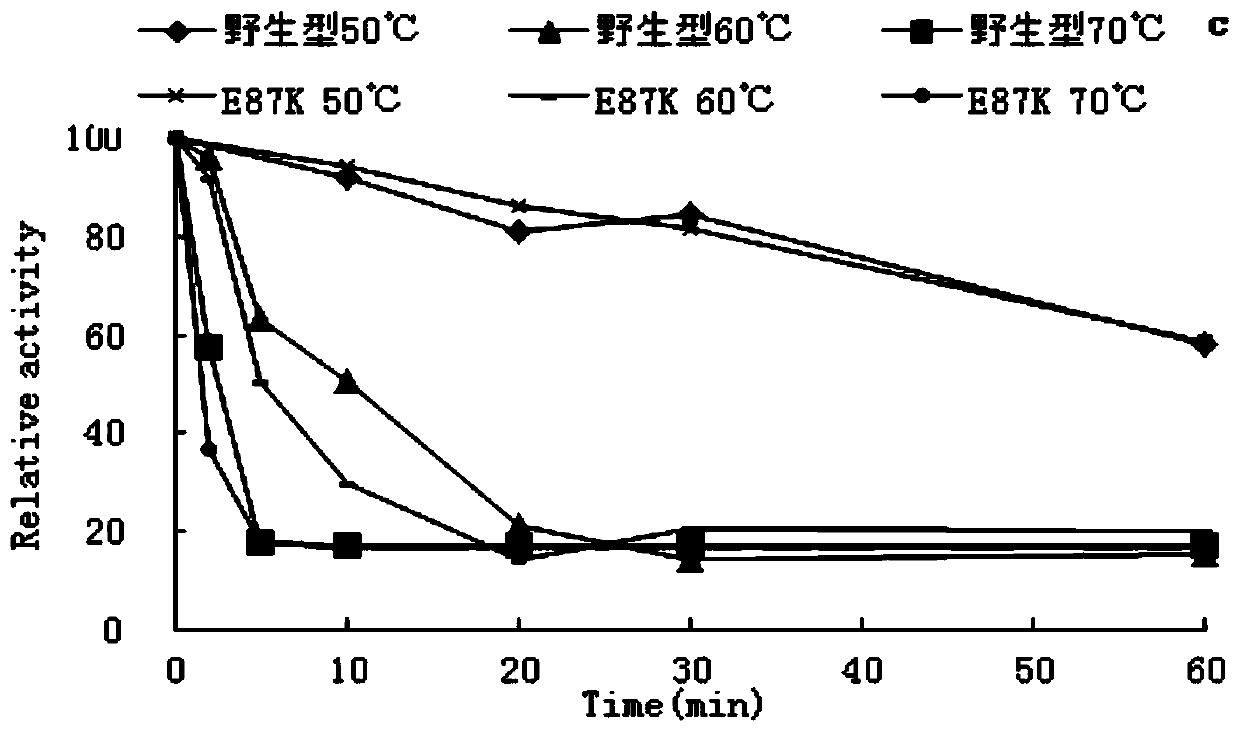
[0054] Example 3 Activity Analysis of Recombinant Amylase Mutant and Female Parent Wild Type
[0055] 1. DNS method: The specific method is as follows: under the given pH and temperature conditions, 1mL of reaction system includes 100μL of appropriate diluted enzyme solution, 900μL of substrate, react for 30min, add 1.5mL of DNS to terminate the reaction, and boil for 5min. After cooling, the OD value was measured at 540 nm. Definition of amylase activity unit: under the condition of 60°C and pH 4.5, the amount of enzyme required to catalyze the hydrolysis of the substrate to release 1 μmol of reducing sugar per minute is an enzyme activity unit.
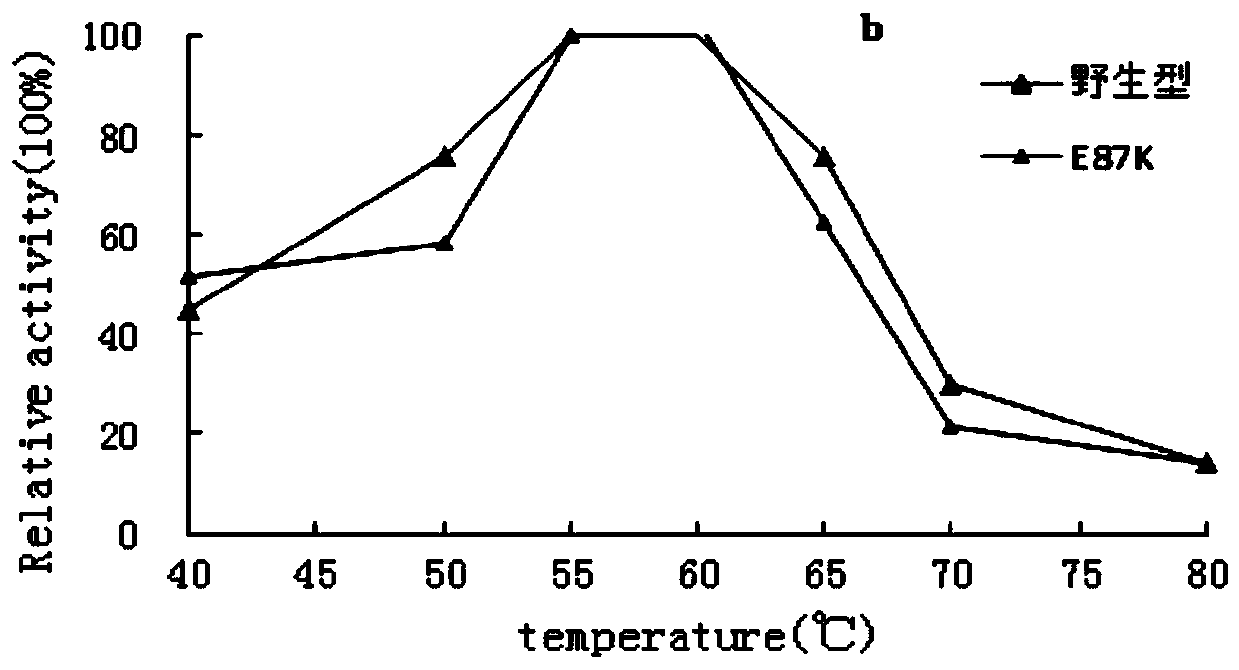
[0056] 2. Determination of the properties of recombinant amylase mutants and maternal wild type
[0057] 1. The optimal pH determination method of the recombinant amylase mutant and the parental wild type is as follows:
[0058] The recombinant amylase mutant purified in Example 2 and the wild type were subjected to enzymatic reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com