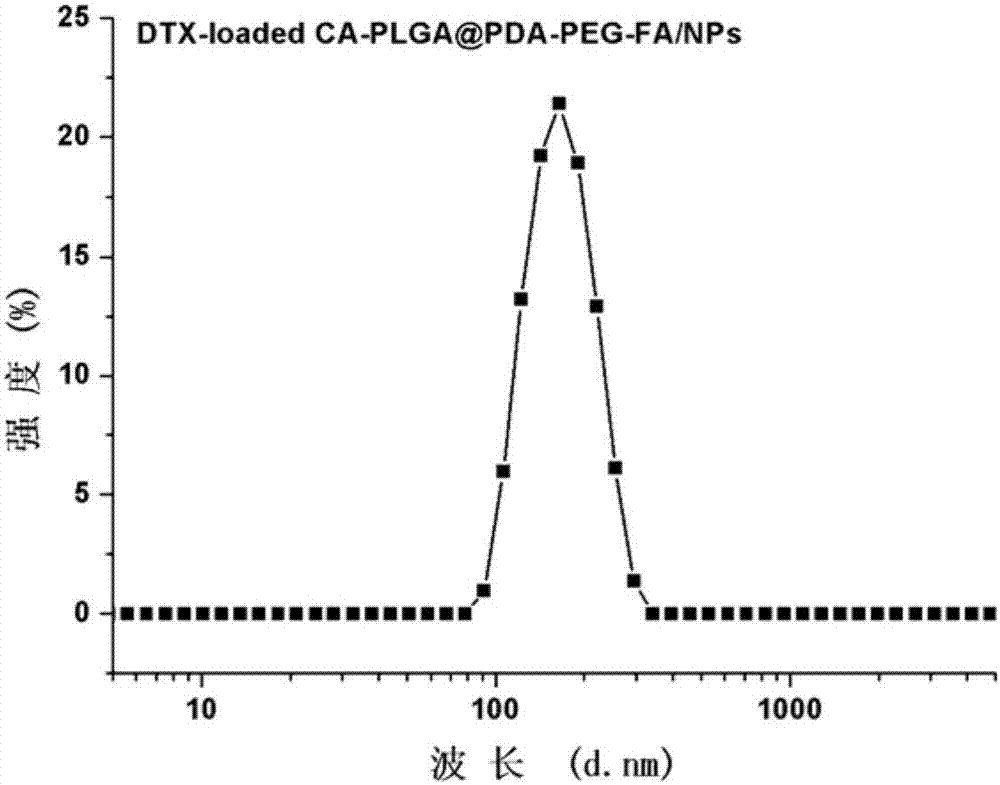
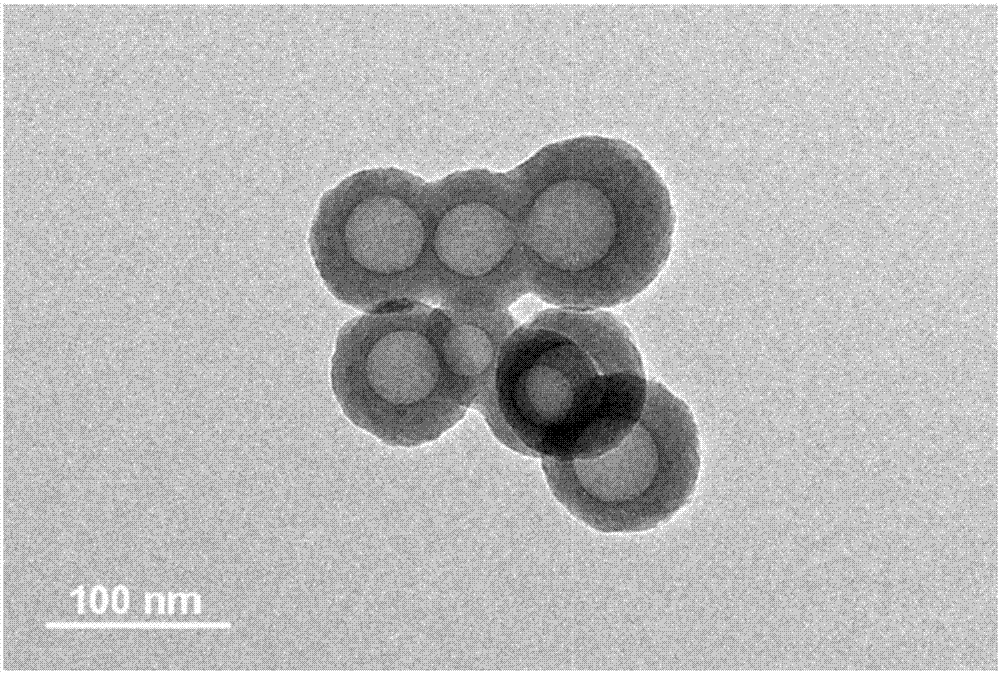
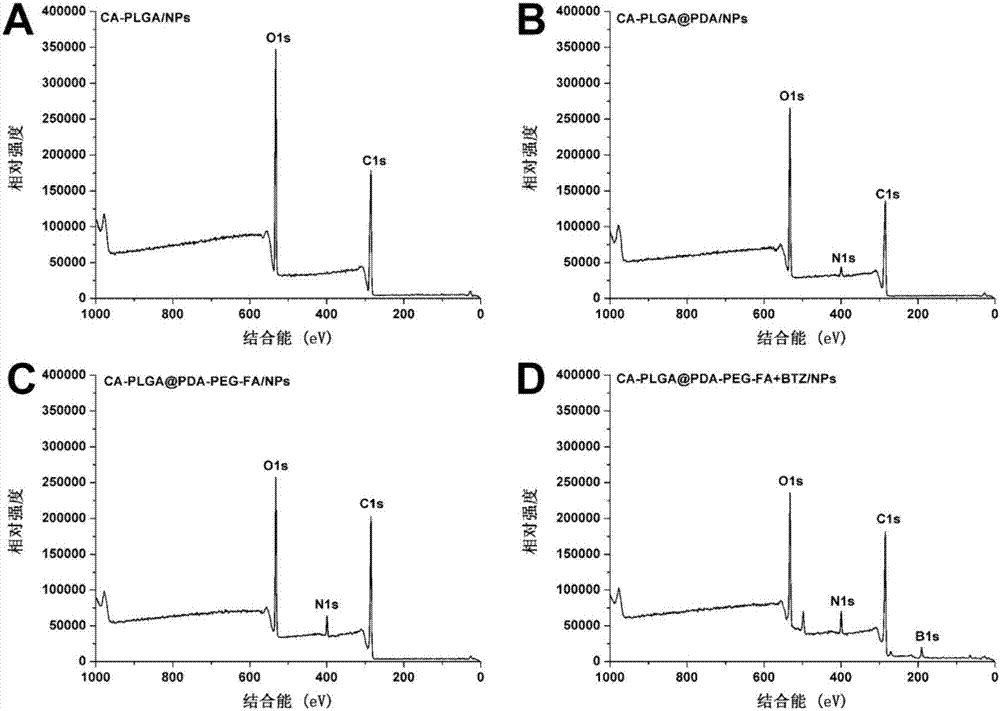
Binary drug loading nanoparticle with pH response and tumor targeting effect as well as preparation method and application thereof
A tumor-targeting and nanoparticle technology, which is applied in the direction of anti-tumor drugs, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of reducing the generality of the method and increasing the difficulty of synthesis, and achieve good therapeutic effects. Method Simple, Adaptable Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A method for preparing folic acid and polydopamine-modified pH-responsive tumor-targeting dual drug-loaded nanoparticles and its application in breast cancer targeted therapy, comprising the following steps:
[0068] (1) Preparation of drug-loaded nanoparticles: weigh 100 mg of cholic acid-polylactic acid-glycolic acid copolymer (CA-PLGA) and 10 mg of docetaxel in proportion, dissolve them in 8 mL of acetone, and stir at room temperature. Add dropwise to 100 mL of 0.03% (w / v) polyethylene glycol vitamin E succinate aqueous solution, continue to stir at room temperature for 12 hours to volatilize acetone, centrifuge at 18,000 rpm for 15 minutes, discard the supernatant, and use deionized water for precipitation Washed 3 times to obtain docetaxel (Docetaxel, DTX)-loaded nanoparticles CA-PLGA / NPs, freeze-dried and set aside;
[0069] (2) Modification of polydopamine: Proportionally, weigh 50 mg of the nanoparticles prepared in (1) and resuspend in 50 mL of 10 mM, pH=8.5 Tr...
Embodiment 2
[0084] A method for preparing folic acid and polydopamine-modified pH-responsive tumor-targeting dual drug-loaded nanoparticles and its application in breast cancer targeted therapy, comprising the following steps:
[0085] (1) Preparation of drug-loaded nanoparticles: weigh 100 mg of cholic acid-polylactic acid-glycolic acid copolymer (CA-PLGA), 2 mg of coumarin-6, dissolve in 8 mL of acetone, and stir at room temperature Add dropwise to 100mL of 0.03% (w / v) polyethylene glycol vitamin E succinate aqueous solution, continue to stir at room temperature for 12 hours to volatilize acetone, centrifuge at 18000rpm for 15 minutes, discard the supernatant, and use the precipitate Washing with deionized water 3 times to obtain nanoparticles CA-PLGA / NPs loaded with coumarin-6 (Coumarin-6, C6), freeze-dried and set aside;
[0086] (2) Modification of polydopamine: Proportionally, weigh 50 mg of the nanoparticles prepared in (1) and resuspend in 50 mL of 10 mM, pH=8.5 Tris buffer, weigh...
Embodiment 3
[0092] A method for preparing folic acid and polydopamine-modified pH-responsive tumor-targeting dual drug-loaded nanoparticles, comprising the following steps:
[0093] (1) Preparation of drug-loaded nanoparticles: Weigh 100 mg of polylactic-co-glycolic acid (PLGA) and 10 mg of paclitaxel in proportion, dissolve them in 10 mL of tetrahydrofuran, and add them dropwise to 100 mL of 0.03 % (w / v) polyethylene glycol vitamin E succinate aqueous solution, continue stirring at room temperature for 12 hours to volatilize tetrahydrofuran, centrifuge at 18000 rpm for 15 minutes, discard the supernatant, and wash the precipitate with deionized water 3 times to obtain paclitaxel (Paclitaxel, PTX) nanoparticles PLGA / NPs, freeze-dried for subsequent use;
[0094] (2) Modification of polydopamine: according to the ratio, weigh 50 mg of the nanoparticles prepared in (1) and resuspend in 50 mL of 10 mM Tris buffer solution with pH=8.5, weigh 10 mg of dopamine hydrochloride, and stir at room t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com