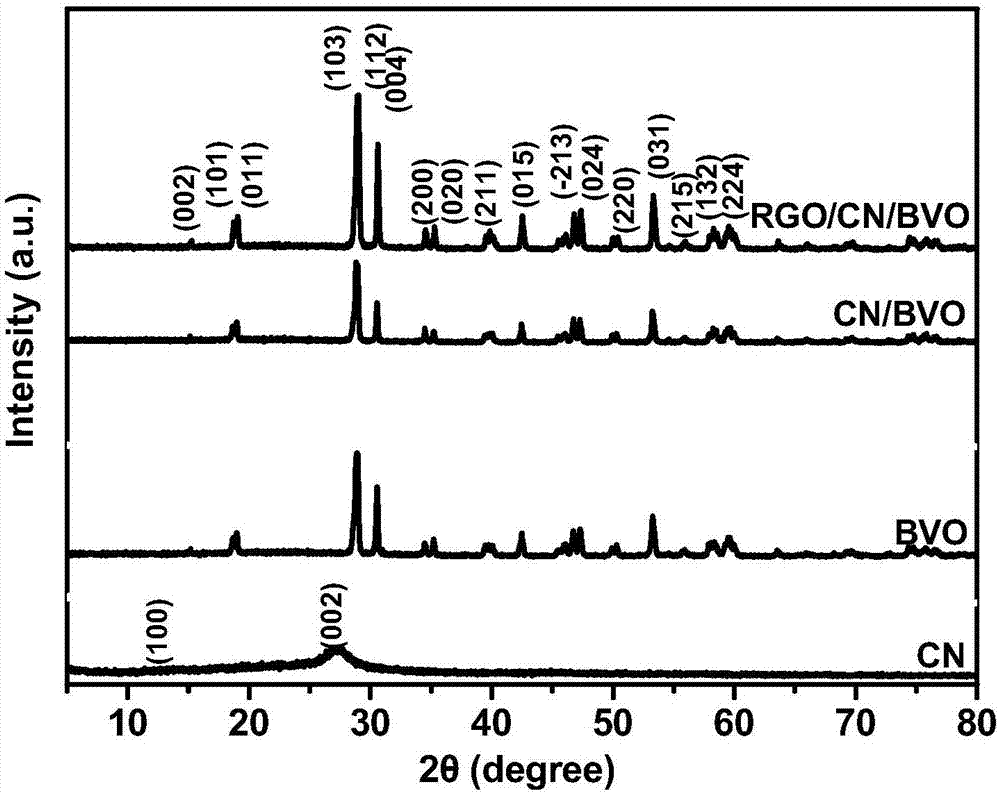
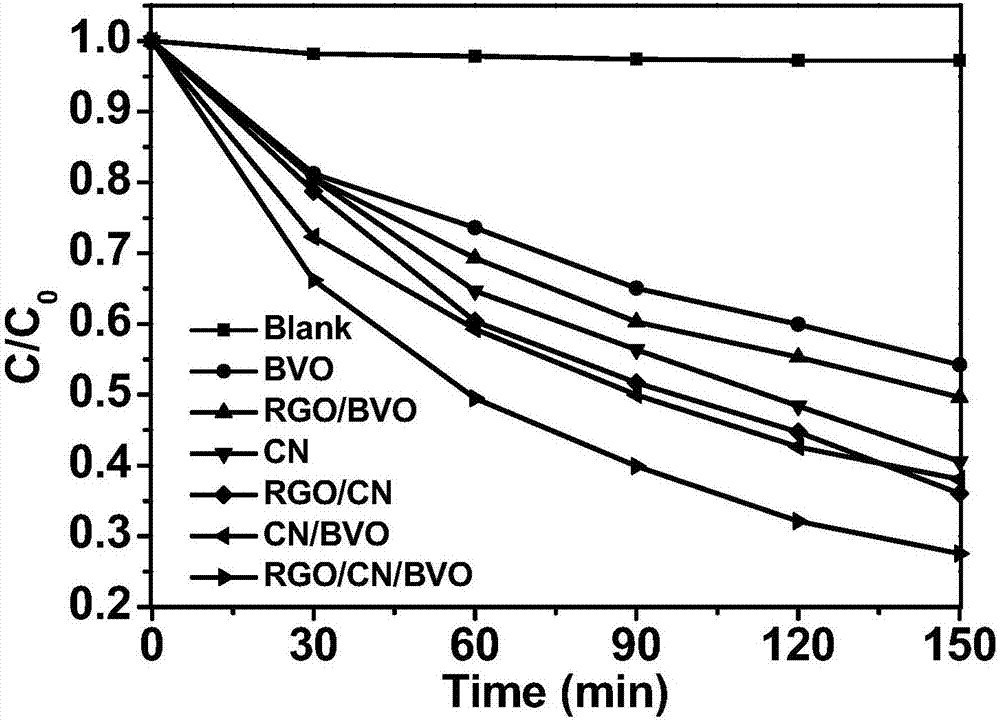
Preparation method and application of reduced graphene oxide/bismuth vanadate/carbon nitride composite material
A technology of graphite phase carbon nitride and composite materials, applied in the field of nanomaterials, can solve the problems of low catalytic efficiency of visible light, and achieve the effects of improving separation efficiency, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Graphite phase nitrogen carbide (g-C 3 N 4 ) Preparation:
[0031] g-C 3 N 4 The preparation adopts the method of thermally polymerizing urea; weigh 10g of urea in a semi-closed crucible, first place it in a 80°C drying oven for 48h to dry the raw materials, and then transfer the crucible to a temperature programmed tube furnace. It is heated to 500°C at a heating rate of 3°C / min for 3h. After natural cooling to room temperature, take it out, grind it to powder with a mortar, and use a concentration of 50mL 0.01mol·L -1 Dilute HNO 3 Wash 3 times to remove residual alkaline species, and then wash 3 times with deionized water and absolute ethanol. Finally, it was dried in an oven at 80°C for 12 hours.
[0032] (2) Preparation of reduced graphene oxide (RGO):
[0033] Firstly prepare graphene oxide (GO), weigh 80mL concentrated sulfuric acid and 0.6g sodium nitrate in a 500mL three-necked flask and stir evenly, ice bath to 0℃, add 1.0g natural flake graphite, and slowly add...
Embodiment 2
[0041] Step (1) and step (2) of this embodiment are the same as in embodiment 1;
[0042] (3) RGO / BVO 4 / g-C 3 N 4 Preparation of composite materials:
[0043] Weigh 1mmol Bi(NO 3 ) 3 ·5H 2 Add O to 0.04mol·L -1 HNO 3 Then add 0.4 g of sodium dodecyl sulfonate (SDS) dissolved in high-purity water to obtain solution A. Weigh 1mmol NH 4 VO 3 Add to NH 3 ·H 2 In O solution, slowly add the prepared solution A to NH while stirring 4 VO 3 In solution, use NH 3 ·H 2 O adjust the pH of the mixed solution to 7, and stir for 90 min to obtain a homogeneous solution. Weigh 100mg g-C 3 N 4 Add 5mg of RGO powder to the above homogeneous solution in sequence, and sonicate for 1h. The obtained solution was transferred to a hydrothermal reaction kettle lined with polytetrafluoroethylene, reacted at 200°C for 20 hours for hydrothermal reaction, centrifuged, washed with water and alcohol for three times, and dried at 60°C for 12 hours.
Embodiment 3
[0045] Step (1) and step (2) of this embodiment are the same as in embodiment 1;
[0046] (3) RGO / BVO 4 / g-C 3 N 4 Preparation of composite materials:
[0047] Weigh 1mmol Bi(NO 3 ) 3 ·5H 2 Add O to 0.04mol L -1 HNO 3 Then add 0.5 g of sodium dodecyl sulfonate (SDS) dissolved in high-purity water to obtain solution A. Weigh 1mmol NH 4 VO 3 Add to NH 3 ·H 2 In O solution, slowly add the prepared solution A to NH while stirring 4 VO 3 In solution, use NH 3 ·H 2 O adjust the pH of the mixed solution to 8, and stir for 90 min to obtain a homogeneous solution. Weigh 100mg g-C 3 N 4 Add 5mg of RGO powder to the above homogeneous solution in sequence, and sonicate for 1h. The obtained solution was transferred to a hydrothermal reactor lined with polytetrafluoroethylene, reacted at 220°C for 20 hours for hydrothermal reaction, centrifuged, washed with water and alcohol three times, and dried at 60°C for 12 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com