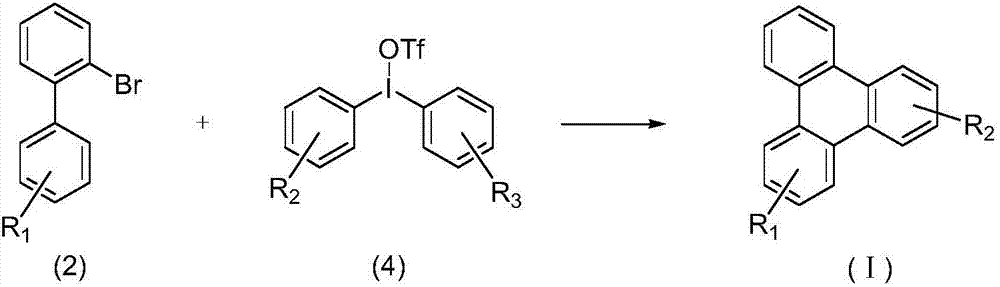
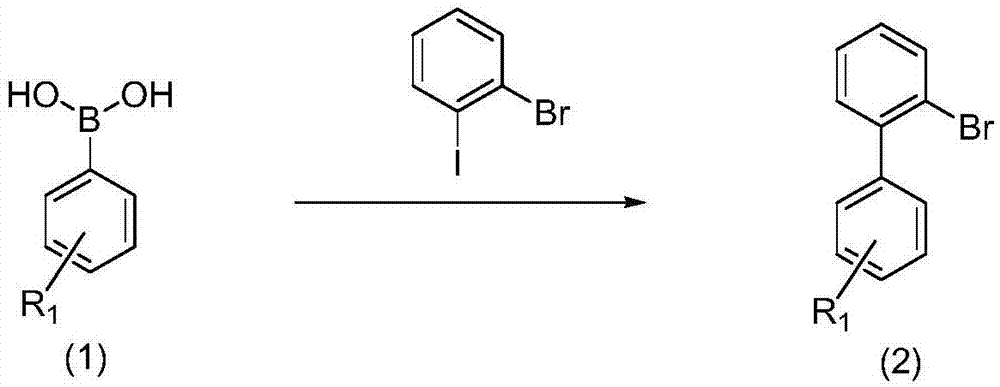
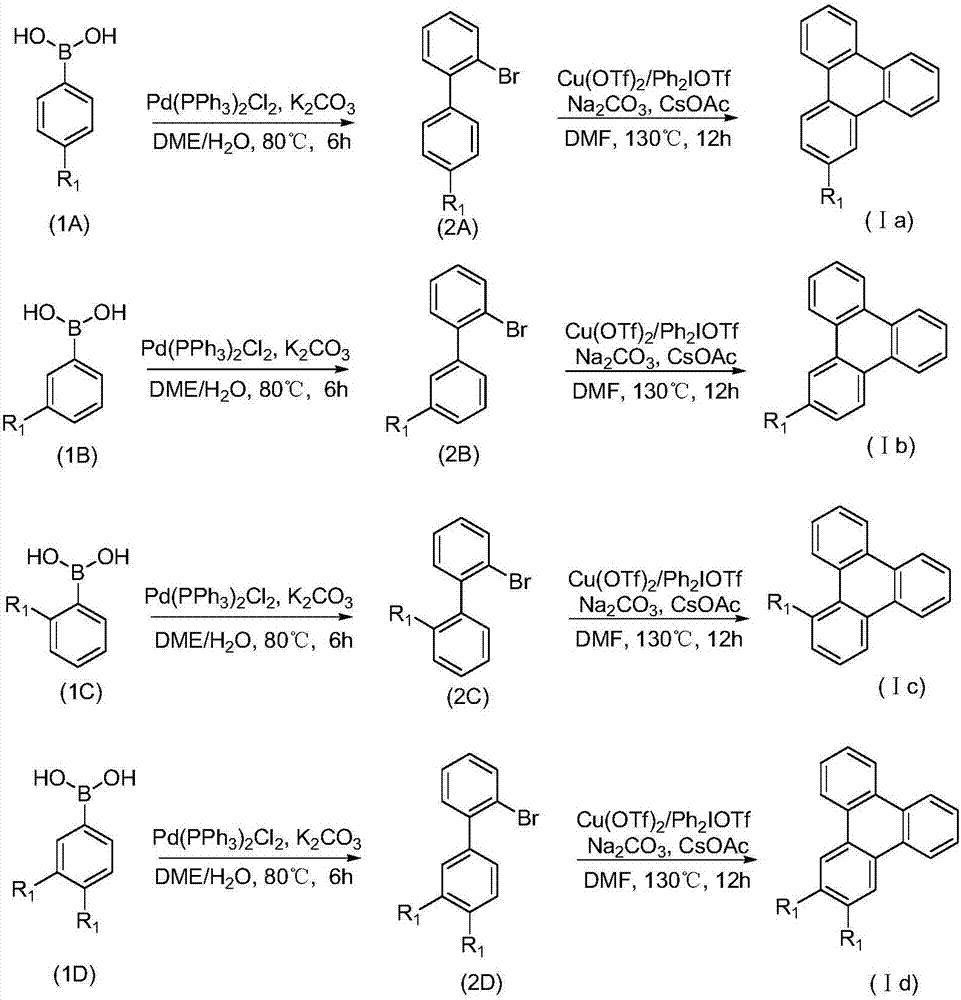
Synthetic method for 9,10-benzophenanthrene compound
A synthesis method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, and hydrocarbon production from halogen-containing organic compounds, etc., can solve the problems of many steps and harsh conditions, and achieve short reaction time, high yield, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Phenylboronic acid (1a, 12mmol, 1.2equiv) and potassium carbonate (15mmol, 1.5equiv) and bistriphenylphosphine palladium dichloride (0.2mmol, 0.02equiv) were placed in a 100mL round bottom flask, and 30mL of DME ( Ethylene glycol dimethyl ether) and 6mL H 2O, add o-bromoiodobenzene (10mmol, 1.0equiv) and heat to 80°C for 6 hours. After the reaction, cool to room temperature, add 50 mL of water to quench the reaction, extract three times with 100 mL of ethyl acetate, dry the organic phase with anhydrous sodium sulfate, filter and spin to dry column chromatography (eluent: PE) to obtain compound 2a.
[0034] N 2 Under protection, in a 25mL Schlenk bottle, add diaryliodonium salt (0.3mmol, 1.5equiv), Cu(OTf) 2 (0.01mmol, 3.61mg), Na 2 CO 3 (0.4mmol, 2.0equiv) and CsOAc (0.6mmol, 3.0equiv), the reaction substrate 2a (0.2mmol, 1.0equiv) and 4mL redistilled DMF were added by syringe, and reacted at 130°C for 12 hours. After the reaction, the solvent was spin-...
Embodiment 2
[0037]
[0038] 4-Methylphenylboronic acid (1b, 12mmol, 1.2equiv) and potassium carbonate (15mmol, 1.5equiv) and ditriphenylphosphine palladium dichloride (0.2mmol, 0.02equiv) were placed in a 100mL round bottom flask, while Add 30mL DME (ethylene glycol dimethyl ether) and 6mL H 2 O, add o-bromoiodobenzene (10mmol, 1.0equiv) and heat to 80°C for 6 hours. After the reaction, cool to room temperature, add 50 mL of water to quench the reaction, extract three times with 100 mL of ethyl acetate, dry the organic phase with anhydrous sodium sulfate, filter and spin dry column chromatography (eluent: PE) to obtain compound 2b.
[0039] N 2 Under protection, in a 25mL Schlenk bottle, add diaryliodonium salt (0.3mmol, 1.5equiv), Cu(OTf) 2 (0.01mmol, 3.61mg), Na 2 CO 3 (0.4mmol, 2.0equiv) and CsOAc (0.6mmol, 3.0equiv) were added to reaction substrate 2b (0.2mmol, 1.0equiv) and 4mL redistilled DMF by syringe, and reacted at 130°C for 12 hours. After the reaction, the solvent was ...
Embodiment 3
[0042]
[0043] 2-Methylphenylboronic acid (1c, 12mmol, 1.2eeqquiv) and potassium carbonate (15mmol, 1.5equiv) and ditriphenylphosphine palladium dichloride (0.2mmol, 0.02equiv) were placed in a 100mL round-bottomed flask while Add 30mL DME (ethylene glycol dimethyl ether) and 6mL H 2 O, add o-bromoiodobenzene (10mmol, 1.0equiv) and heat to 80°C for 6 hours. After the reaction, cool to room temperature, add 50 mL of water to quench the reaction, extract three times with 100 mL of ethyl acetate, dry the organic phase with anhydrous sodium sulfate, filter and spin to dry column chromatography (eluent: PE) to obtain compound 2c.
[0044] N 2 Under protection, in a 25mL Schlenk bottle, add diaryliodonium salt (0.3mmol, 1.5equiv), Cu(OTf) 2 (0.01mmol, 3.61mg), Na 2 CO 3 (0.4mmol, 2.0equiv) and CsOAc (0.6mmol, 3.0equiv) were added to reaction substrate 2c (0.2mmol, 1.0equiv) and 4mL redistilled DMF with a syringe, and reacted at 130°C for 12 hours. After the reaction was com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com