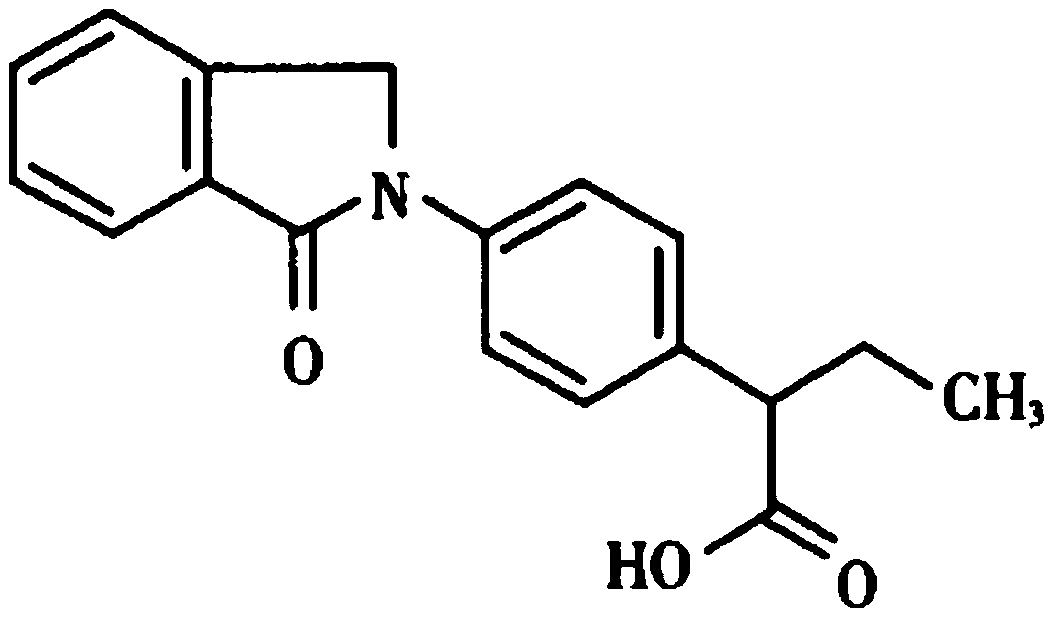
Indobufen pharmaceutical composition and its quality control method
A technology of indobufen and its composition, which is applied in the field of medical technology, and can solve problems such as low dissolution rate and slow drug speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1: Preparation of indobufen tablets
[0120] prescription:
[0121] Indobufen 200mg,
[0122] Lactose 40mg,
[0123] Microcrystalline Cellulose 25mg,
[0124] Croscarmellose sodium (disintegrant) 10mg,
[0125] Polyvinylpyrrolidone (binder) 5 mg,
[0126] Micropowder silica gel (flow aid) 2mg,
[0127] Magnesium stearate (lubricant) 2mg.
[0128] Preparation method:
[0129] (1) After mixing indobufen and sugar diluent, carry out pretreatment together (spray into water accounting for 10w / w% of the weight of the powder material and seal it for 18 hours); the rest of the solid materials are respectively pulverized into 80-mesh powder;
[0130] (2) Mix the pretreatment mixture obtained in step (1) with a cellulose diluent and a disintegrant accounting for 40% of the total amount;
[0131] (3) Add a binder solution (mixed with water to a concentration of 4w / v%) in advance with a solvent in the mixed material obtained in step (2), and prepare wet granules...
Embodiment 2
[0133] Embodiment 2: preparation of indobufen tablets
[0134] prescription:
[0135] Indobufen 200mg,
[0136] Lactose 30mg,
[0137] Microcrystalline Cellulose 40mg,
[0138] Crospovidone (disintegrant) 5mg,
[0139] Polyvinylpyrrolidone (binder) 8 mg,
[0140] Micropowder silica gel (flow aid) 1mg,
[0141] Magnesium stearate (lubricant) 5mg.
[0142] Preparation method:
[0143] (1) After mixing indobufen and carbohydrate diluent, carry out pretreatment together (spray into water accounting for 9w / w% of powder material weight and seal and let stand for 16 hours); the remaining solid materials are respectively crushed into 80-mesh powder;
[0144] (2) Mix the pretreatment mixture obtained in step (1) with the cellulose diluent and the disintegrant accounting for 60% of the total amount;
[0145] (3) Add a binder solution (prepared to 5w / v% concentration with 40% ethanol solution) in the mixed material obtained in step (2) with a solvent in advance, and prepare we...
Embodiment 3
[0147] Embodiment 3: Preparation of indobufen tablets
[0148] prescription:
[0149] Indobufen 200mg,
[0150] Lactose (anhydrous lactose) 50mg,
[0151] Microcrystalline Cellulose 15mg,
[0152] Sodium starch glycolate (disintegrant) 15mg,
[0153] Hypromellose (binder) 3mg,
[0154] Talc powder (glidant) 5mg,
[0155] Stearic acid (lubricant) 1mg.
[0156] Preparation method:
[0157] (1) After mixing indobufen and carbohydrate diluent, carry out pretreatment together (spray into water accounting for 8w / w% of the weight of the powder material and seal it for 20 hours); the remaining solid materials are respectively pulverized into 80-mesh powder;
[0158] (2) Mix the pretreatment mixture obtained in step (1) with the cellulose diluent and the disintegrant accounting for 50% of the total amount;
[0159] (3) Add the binder solution (mixed to 3w / v% concentration with 70% ethanol solution) in the mixed material obtained in step (2) with a solvent in advance, and pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com