Application of Corylifol A in preparing anti-radiation drug
An anti-radiation and drug technology, applied in the field of anti-radiation medicine, can solve the problems of weak radiotherapy selectivity, affecting the treatment effect of tumor patients, and treatment failure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Extraction of Corylifol A and its characterization
[0029] Extraction of Corylifol A
[0030] 15 kg of psoralen fruit, crushed, soaked and extracted with 75% ethanol at room temperature for 3 times, each time for 24 to 36 hours, concentrated under reduced pressure to obtain 5 L of extract, and the extract was applied to silica gel column chromatography, with a volume ratio of 100:1 Gradient elution to 0:100 petroleum ether-ethyl acetate system to obtain 259g of extract at 5:5; the above extract was subjected to silica gel column chromatography, and dichloromethane- Gradient elution with ethyl acetate system, detection by thin layer chromatography, 5 g of fractions containing Corylifol A were collected, and then preparative liquid chromatography was eluted with a methanol-water system with a volume ratio of 75:25 to 95:5 to obtain Pure Corylifol A 1.6g.
[0031] Characterization of Corylifol A
[0032] Pale yellow powder, by electrospray ion mass spectromet...
Embodiment 2
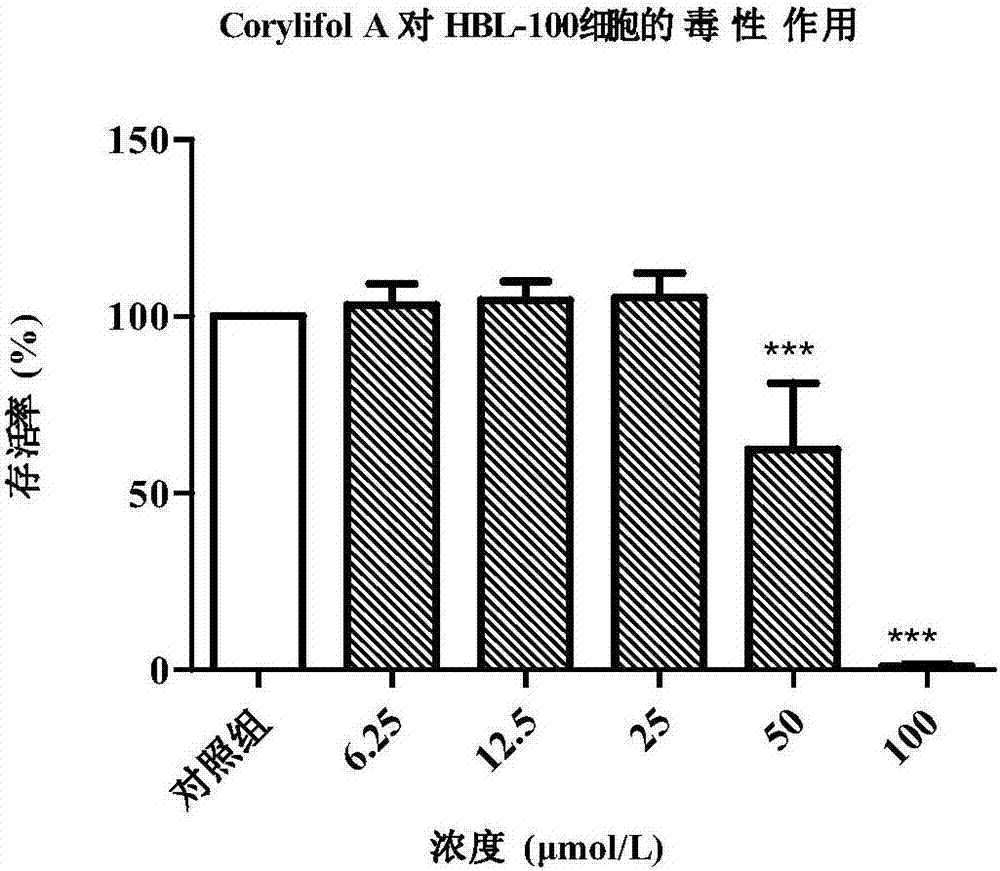
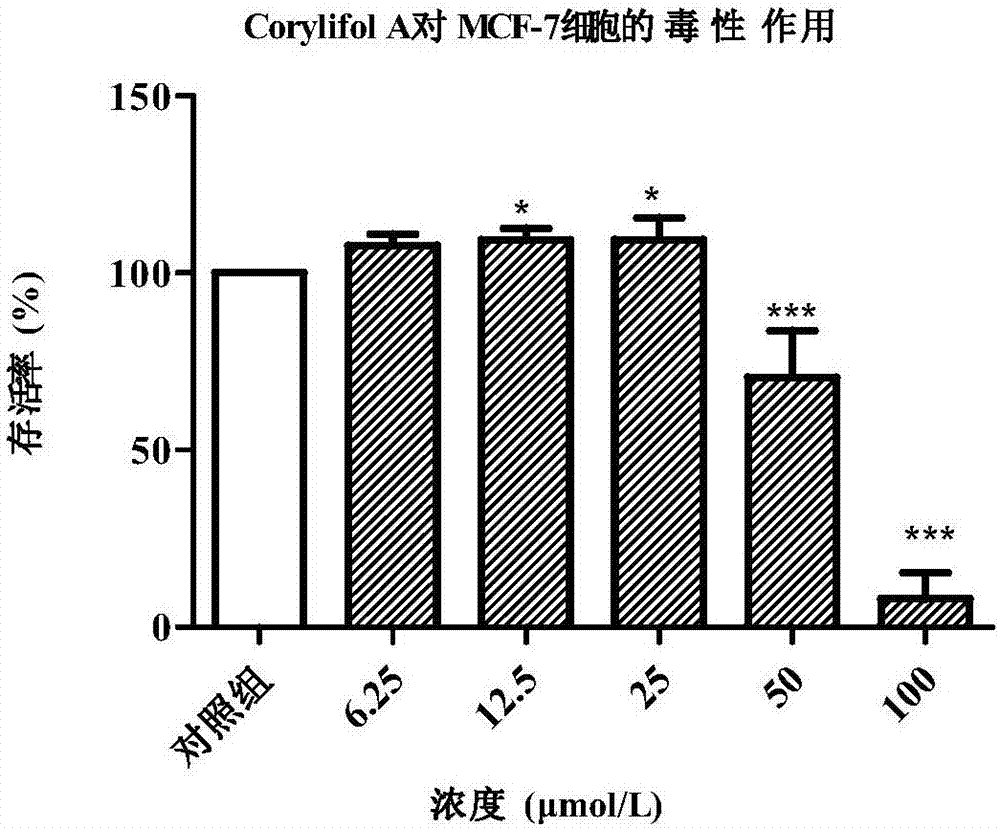
[0034]Example 2 In vitro cytotoxicity test of Corylifol A
[0035] 2.1 Cell culture
[0036] HBL-100 cells (ATCC) in RPMI-1640 medium (containing 10% fetal bovine serum, 100U / mL penicillin and 0.1mg / mL streptomycin), MCF-7 cells (ATCC) in DMEM / High Gluose medium (containing 10% fetal bovine serum, 100 U / mL penicillin and 0.1 mg / mL streptomycin), at 37°C, 5% CO 2 , cultured in an incubator with saturated humidity. When the cells adhere to the wall and grow to a density of 70-80%, they are subcultured at a ratio of 1 / 2. The number of cells in good growth phase was used for experiments.
[0037] 2.2 Cytotoxicity experiment
[0038] CCK-8 method was used to test the effect of test drugs on the viability of HBL-100 and MCF-7 cells.
[0039] (1) Cell inoculation: cells in good logarithmic growth phase were taken, digested with 0.25% trypsin and prepared into cell suspension, and 7×10 3 Cells / well, 100 μL of cell suspension was uniformly inoculated in a 96-well plate (the periph...
Embodiment 3
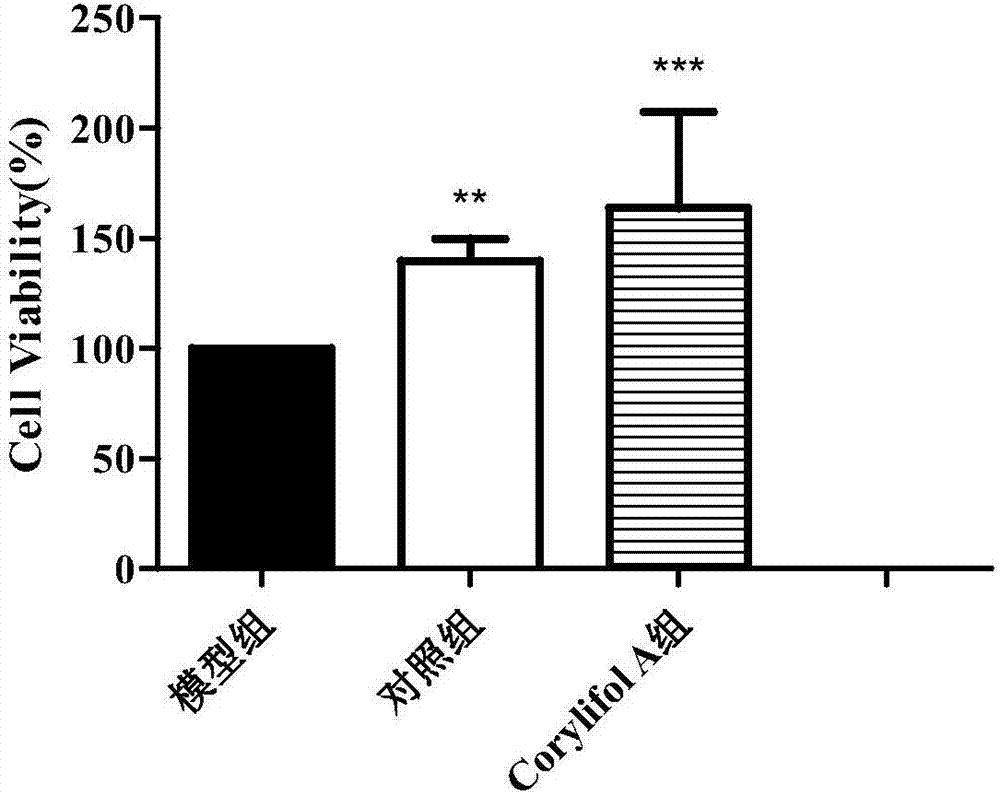
[0047] Example 3 Cell Anti-radiation Experiment of Corylifol A
[0048] 3.1 Cell radiation resistance experiment
[0049] Under 2.2 of Example 2, under the condition that there is no significant difference in cell viability between the corylifol A group and the control group, 25 μmol / L was selected to test its radiation damage repairing effect on HBL-100 and MCF-7 cells. The experiment was divided into blank group (no irradiation, no administration), control group (irradiation, no administration), and corylifol A group (ie, experimental group, irradiation, administration).
[0050] Get the cells with good logarithmic growth phase cultured in 2.1 of Example 2, digest them with 0.25% trypsin and prepare a cell suspension, and prepare 7×10 3 1 / well, 100 μL of cell suspension was uniformly inoculated in a 96-well plate (the periphery of the experimental well was filled with sterile PBS); after culturing in the incubator for 12 h, the 96-well plate was taken out, and 100 μL of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com