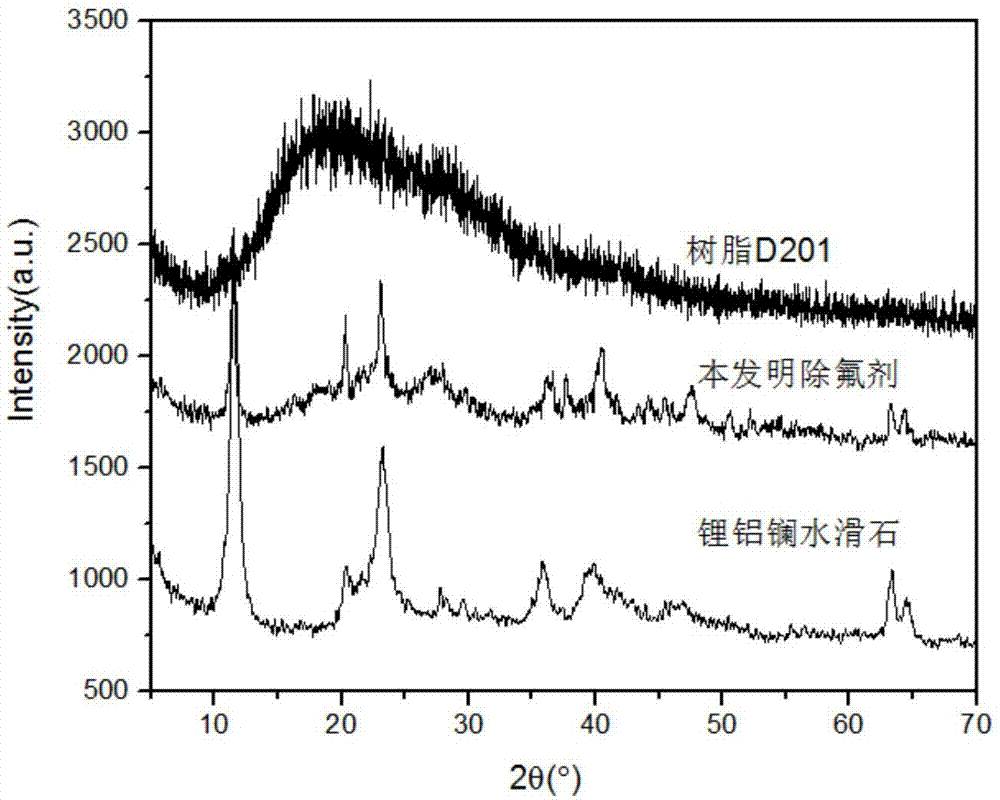
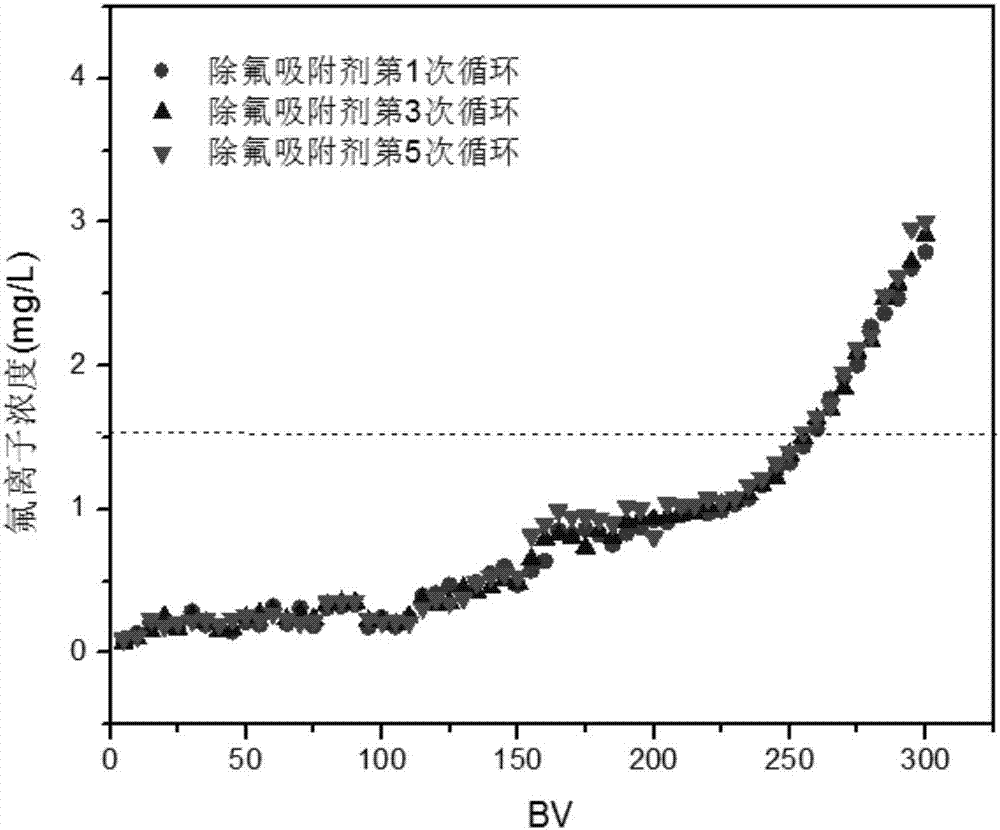
Defluorination adsorbent and preparation method thereof
A technology of adsorbent and exchange resin, which is applied in the field of fluorine removal adsorbent and its preparation, can solve the problems of active component loss, difficult industrial application, poor mechanical strength, etc., and achieve faster adsorption speed, stable performance and strong mechanical strength. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Salt solution preparation: 8.7g of lanthanum chloride heptahydrate and 12.15g of aluminum chloride hexahydrate were added to 120ml of deionized aqueous solution containing 30ml of ethanol solution, and ultrasonically dissolved to obtain a clear solution.
[0038] (2) Reaction: Add the clear solution obtained in step (1) into a reactor equipped with a stirring and temperature control device, add 20ml of anion exchange resin D201, and stir at a temperature of 60° C. for 7 hours. Filter out the resin, add 15% sodium hydroxide solution, and stir for 4h. Filter out the resin and set aside.
[0039] (3) Add lithium salt: prepare 10.5% lithium chloride solution, add the resin filtered out in step (2), and crystallize in an oven at 60° C. for 5 hours.
[0040] (4) Post-treatment: filter out the resin treated in step (3), and wash with water to obtain the fluorine-removing adsorbent.
[0041] As a comparison, lithium aluminum lanthanum hydrotalcite was prepared by co-preci...
Embodiment 2
[0045] (1) Salt solution preparation: Weigh 9.8g of lanthanum nitrate hexahydrate and 28.1g of aluminum nitrate nonahydrate and add them to 260ml of deionized aqueous solution containing 50ml of ethanol solution, ultrasonically dissolve them to obtain a clear solution.
[0046] (2) Reaction: Add the clear solution obtained in step (1) into a reactor equipped with a stirring and temperature control device, add 40 ml of anion exchange resin D201, and stir at a temperature of 80° C. for 10 h. Filter out the resin, add 20% sodium hydroxide solution, and stir for 5h. Filter out the resin and set aside.
[0047] (3) Add lithium salt: prepare 12% lithium salt solution, add the resin filtered out in step (2), and crystallize in an oven at 80° C. for 4 hours.
[0048] (4) Post-treatment: filter out the resin treated in step (3), and wash with water to obtain the fluorine-removing adsorbent.
[0049] Test its adsorption capacity according to the method of Example 1.
Embodiment 3
[0051] (1) Salt solution preparation: 12.7g of lanthanum chloride heptahydrate and 15.15g of aluminum chloride hexahydrate were added to 120ml of deionized aqueous solution containing 30ml of ethanol solution, and ultrasonically dissolved to obtain a clear solution.
[0052] (2) Reaction: Add the clear solution obtained in step (1) into a reactor equipped with a stirring and temperature control device, add 15ml of anion exchange resin D202, and stir at a temperature of 80° C. for 4 hours. Filter out the resin, add 12% sodium hydroxide solution, and stir for 3h. Filter out the resin and set aside.
[0053] (3) Add lithium salt: prepare 12.5% lithium chloride solution, add the resin filtered out in step (2), and crystallize in an oven at 60° C. for 5 hours.
[0054] (4) Post-treatment: filter out the resin treated in step (3), and wash with water to obtain the fluorine-removing adsorbent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com