Preparation method of 1-(4-chlorphenyl)-2-cyclopropyl-1-acetone
A technology of cyclopropylmethyl ketone and p-chlorobenzaldehyde, which is applied in the efficient preparation of 1--2-cyclopropyl-1-propanone, and in the field of safety, can solve problems such as high cost, difficult raw materials, and high toxicity, and achieve The effects of mild reaction conditions, controllable process and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
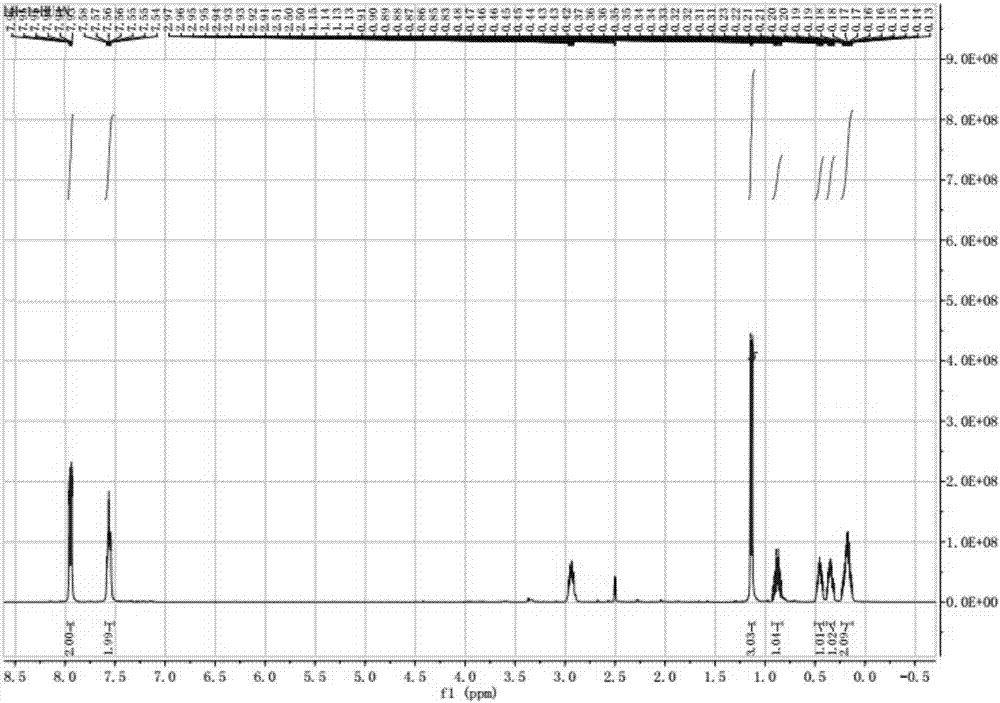
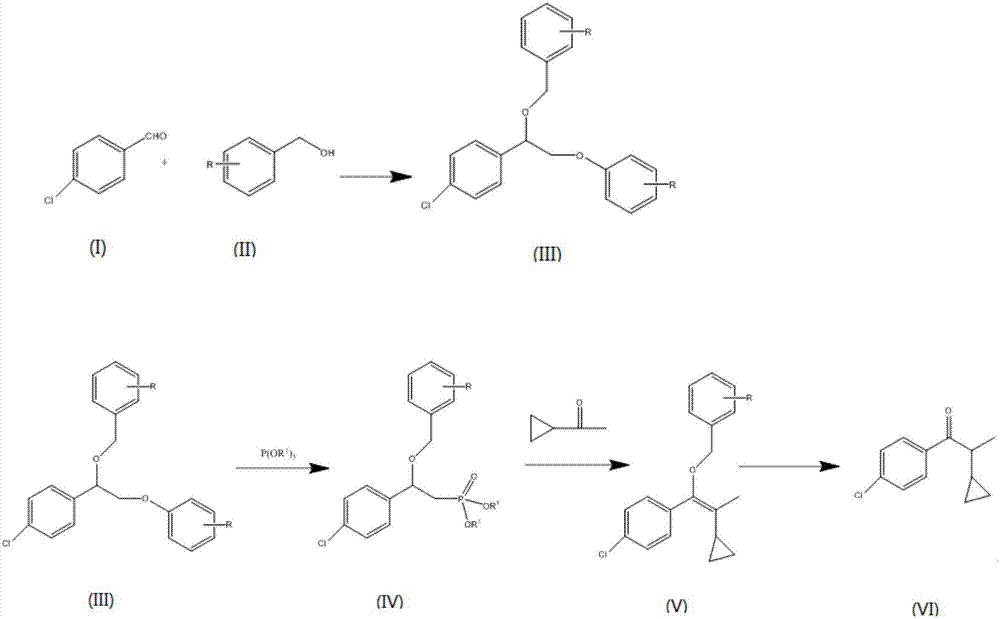
[0041] Embodiment 1: the preparation of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (VI), (the compound of formula (II) is benzyl alcohol, R is hydrogen):
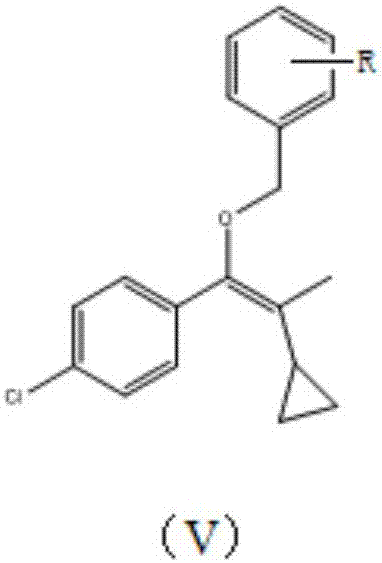
[0042] Throw in benzyl alcohol (II) and trimethyl orthoformate catalyst, add p-chlorobenzaldehyde dropwise after heating up to 68°C, the dropping time lasts for 4 hours, keep warm for 2 hours after the addition, take a sample for detection, when p-chlorobenzaldehyde< At 0.5%, the catalyst trimethyl orthoformate is recovered by distillation to obtain a chlorobenzaldehyde dimethyl acetal (III) solution; 2-methyltetrahydrofuran is added to the chlorobenzaldehyde dimethyl acetal (III) solution, and at 65° C. Add trimethyl phosphite dropwise, and react under the catalysis of boron trifluoride to generate α-benzyloxy dimethyl p-chlorobenzyl phosphonate. When the reaction is over, cool down to 35°C and add tetrabutylammonium bromide, t- Sodium butoxide and 20wt% LiBr, and cyclopropyl methyl ketone is added dropwise at this tempe...
Embodiment 2
[0044] Embodiment 2: the preparation of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone, (the compound of formula (II) is p-chlorobenzyl alcohol, R is chlorine):
[0045] Put in p-chlorobenzyl alcohol (II) and trimethylchlorosilane catalyst, add p-chlorobenzaldehyde dropwise after heating up to 65°C, the dropping time lasts for 3 hours, keep warm for 1.5 hours after the dropping, take samples for detection, when p-chlorobenzaldehyde When benzaldehyde<0.5%, distillation reclaims catalyst trimethylchlorosilane, obtains chlorobenzaldehyde dimethyl acetal (III) solution; In chlorobenzaldehyde dimethyl acetal (III) solution, add N-methylpyrrolidone, in Add trimethyl phosphite dropwise at 60°C, react under the catalysis of niobium pentachloride to generate α-benzyloxy dimethyl p-chlorobenzylphosphonate, when the reaction is over, cool down to 30°C and add benzyltriethyl Ammonium chloride, sodium methoxide and 15wt% LiCl, and cyclopropyl methyl ketone was added dropwise at this temperat...
Embodiment 3
[0046] Embodiment 3: the preparation of 1-(4-chlorophenyl)-2-cyclopropyl-1-propanone (VI), (the compound of formula (II) is p-methyl benzyl alcohol, R is methyl):
[0047] Throw in p-methyl benzyl alcohol (II) and trimethyl orthoformate catalysts, add p-chlorobenzaldehyde drop by drop after heating up to 70°C, the dropping time lasts for 4 hours, keep warm for 3 hours after the dropping, take samples for detection, when p-chlorobenzaldehyde When benzaldehyde<0.5%, distillation reclaims catalyst trimethyl orthoformate, obtains chlorobenzaldehyde dimethyl acetal (III) solution; In chlorobenzaldehyde dimethyl acetal (III) solution, add dimethyl formamide, in Add trimethyl phosphite dropwise at 70°C, react under the catalysis of aluminum chloride to generate α-benzyloxy-p-chlorobenzyl phosphonic acid dimethyl, when the reaction is over, cool down to 40°C and add tetrabutylammonium chloride , lithium diisopropylamide and 25wt% LiCl, and dropwise add cyclopropyl methyl ketone at thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com