Denitration catalyst and preparation method thereof
A technology of denitration catalyst and co-catalyst group, applied in the field of inorganic new materials, can solve the problems of unstable activity and unbalanced active center, and achieve the effect of resisting uneven deposition, uniform dispersion and good catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
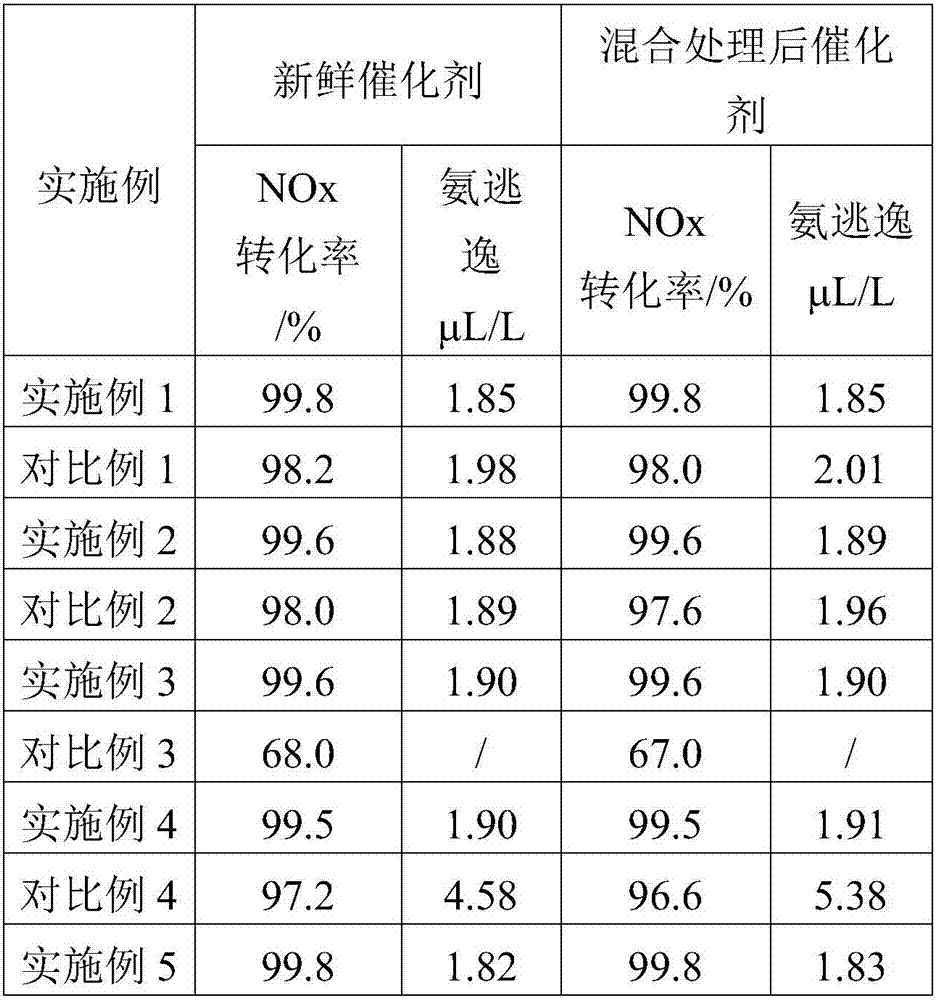
[0038] will contain TiO 2 500g of titanyl sulfate is dissolved in the sulfuric acid solution, adding 3 A total of 22.5g of ammonium metatungstate and 5g of ammonium fluoride solution in terms of F form a TiO-containing 2 It is a 35g / L mixed solution. After stirring for 2 hours, gradually add ammonia water to adjust the pH value to 9.5. After the precipitation is complete, filter and wash; then use deionized water to make a slurry with a water content of 50%, and add V 2 o 5 A total of 12.5g of ammonium metavanadate solution, stirred for 1.5h, dried directly, and roasted at 600°C for 8h; after roasting, the powder was mixed with WO 3 A total of 7.5g of ammonium metatungstate solution was made into a slurry with a water content of 30%. After stirring, 4g of polyethylene oxide was added, stirred for 40min, sealed and left for 24h, dried, and roasted at 600°C for 8h to obtain a denitration catalyst. The fresh catalyst obtained above and the catalyst mixed with the catalytic cra...
Embodiment 2
[0042] will contain TiO 2 500g of titanyl sulfate is dissolved in the sulfuric acid solution, and the 3 20g of ammonium molybdate and 4g of ammonium fluoride solution in terms of F to form TiO 2 It is a 35g / L mixed solution. After stirring for 2 hours, gradually add ammonia water to adjust the pH value to 8.5. After the precipitation is complete, filter and wash; then use deionized water to make a slurry with a water content of 50%, and add V 2 o 5 A total of 11.5g of ammonium metavanadate solution, stirred for 1.5h, dried directly, and roasted at 600°C for 8h; after roasting, the powder was mixed with MoO 3 A total of 7.5g of ammonium molybdate solution was made into a slurry with a water content of 30%. After stirring, 4g of polyethylene oxide was added, stirred for 40 minutes, sealed and left for 24 hours, dried, and roasted at 600°C for 8 hours to obtain a denitration catalyst. The fresh catalyst obtained above and the catalyst mixed with the catalytic cracking vanadium...
Embodiment 3
[0046] will contain TiO 2 500g of titanyl sulfate is dissolved in the sulfuric acid solution, and the 3 20g of ammonium molybdate, in WO 3 20g of ammonium metatungstate and 4g of ammonium fluoride solution in terms of F to form TiO-containing 2 It is a 35g / L mixed solution. After stirring for 2 hours, gradually add ammonia water to adjust the pH value to 8.5. After the precipitation is complete, filter and wash; then use deionized water to make a slurry with a water content of 50%, and add V 2 o 5 A total of 10g of ammonium metavanadate solution, stirred for 1.5h, dried directly, and roasted at 600°C for 8h; after roasting, the powder was mixed with MoO 3 A total of 6g of ammonium molybdate solution was used to make a slurry with a water content of 30%. After stirring, 4g of polyethylene oxide was added, stirred for 40 minutes, sealed and left for 24 hours, dried, and roasted at 600°C for 8 hours to obtain a denitration catalyst. The fresh catalyst obtained above and the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com