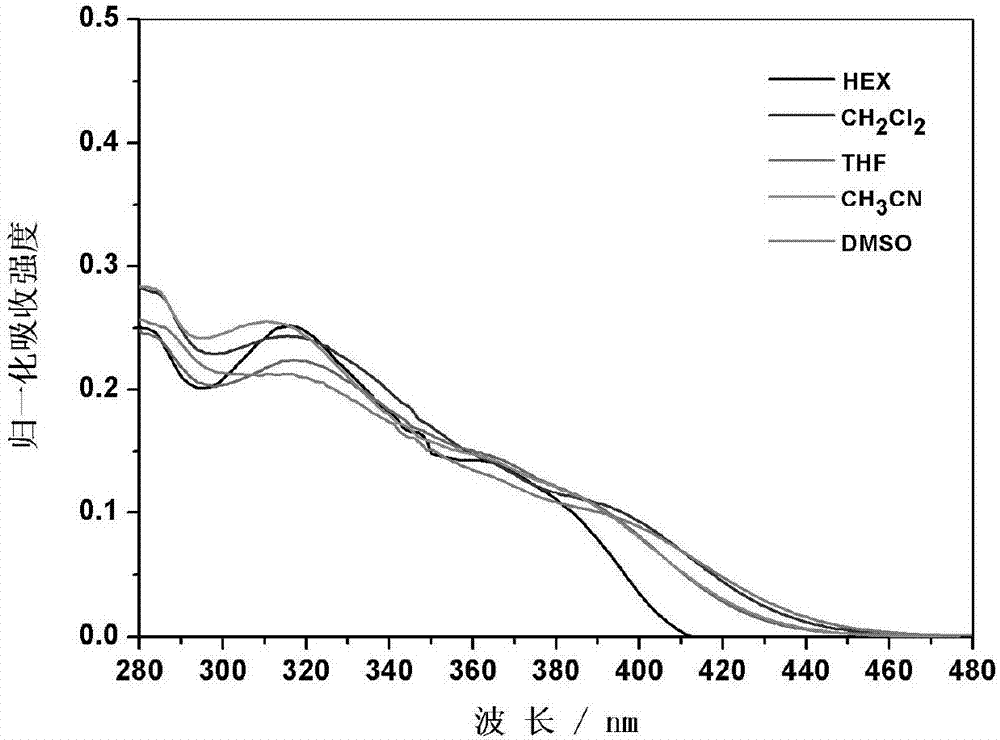
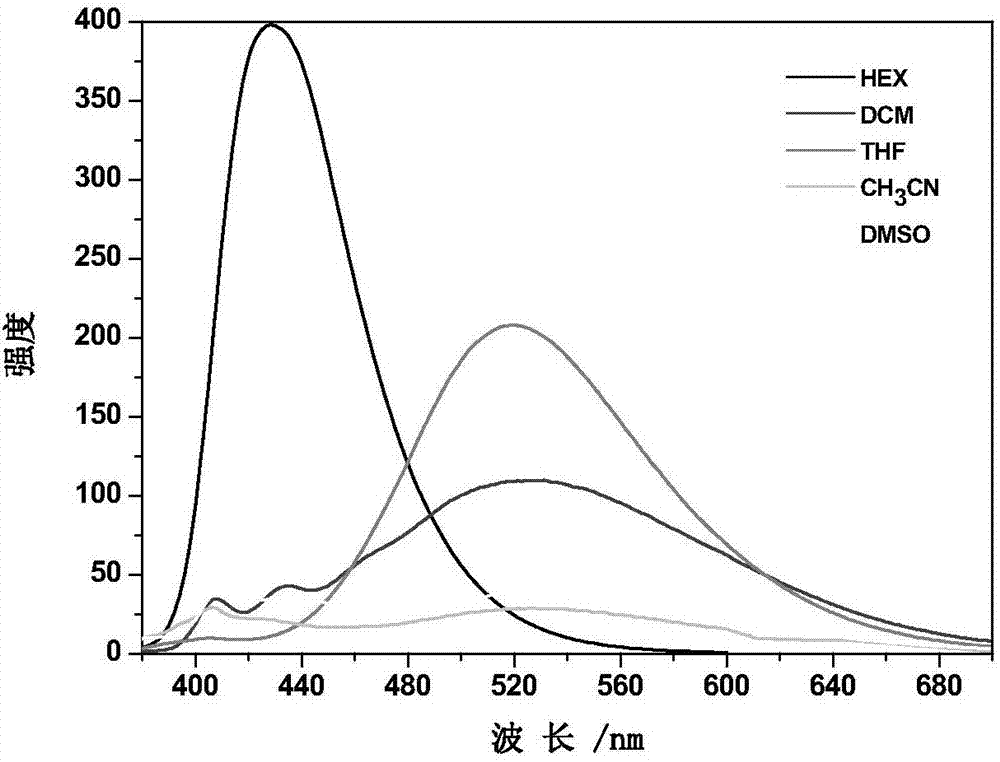
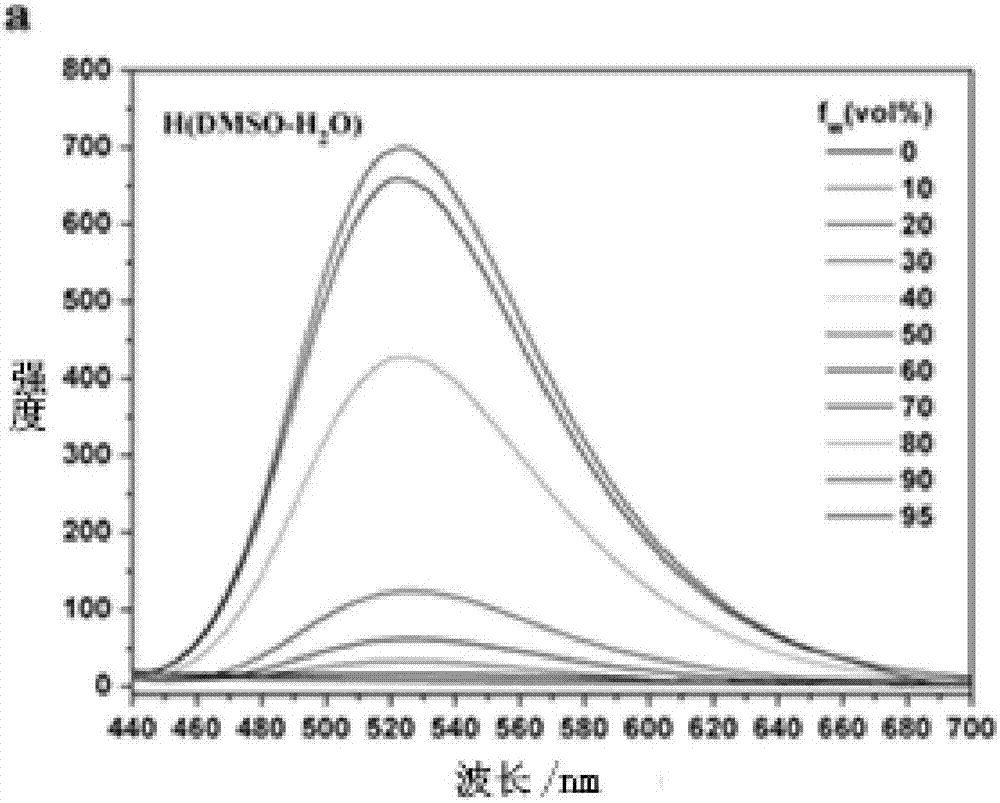
Carbazole compound containing thianthrene and diphenylethlene structures and preparation of carbazole compound
A technology of diphenylethylene and thiaanthracene tetroxide, which is applied in chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems of non-luminescence, luminescence, quenching, etc., and achieve high luminous efficiency and easy operation Good performance and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. The preparation of 9-ethyl-3-(1,2-diphenylvinyl)-6-(5,5,10,10-tetraoxythianthracenyl)carbazole, the steps are as follows:
[0034] (1) Synthesis of 9-ethyl-6-bromo-3-carbazolylbenzophenone;
[0035] In a 250 mL vessel with a stirrer, add 9-ethyl-3-bromo-carbazole (3.57 g, 13.03 mmol), benzoyl chloride (1.8 mL, 2.19 g, 15.64 mmol) and 60 mL of CH 2 Cl 2; then under nitrogen atmosphere, aluminum chloride (1.82g, 13.67mmol) was slowly added to the above mixture; the reaction was maintained at 40°C for 6 hours; after cooling to room temperature, the mixture was poured into 200mL of cold water and washed with 50mL of CH 2 Cl 2 Extracted 3 times; the organic layer was washed with water, dried over anhydrous magnesium sulfate, and the solvent was evaporated; the crude product was purified by silica gel column chromatography using petroleum ether / dichloromethane as eluent to give 9-ethyl-6-bromo - 4.0 g of 3-carbazolylbenzophenone, the yield is 81%.
[0036] (2) ...
Embodiment 2
[0045] Embodiment 2. Structure, preparation and performance of organic electroluminescent devices:
[0046] The device we prepared is a blue-green organic electroluminescent device, and the device structure is: ITO / NPB (thickness 50nm) / CPB (thickness 50nm) / compound DPECTT (thickness 50nm) / Bphen (thickness 20nm) / LiF (thickness 0.5nm) / Al (thickness 150nm), wherein, NPB is used as a hole transport material; CPB is used as an electron blocking material; 9-ethyl-3-(1,2-diphenylvinyl)-6-(5,5,10, 10-Tetraoxythianthranyl)carbazole (compound DPECTT) was used as the light-emitting layer; Bphen was used as the electron-transporting layer.
[0047] The preparation steps of the device: clean the ITO glass, first scrub the ITO glass with acetone, then rinse it with water, then ultrasonically clean it with a cleaning solution, and then ultrasonically clean it with water; dry the cleaned substrate with nitrogen, and finally use ultraviolet- Ozone treatment to fully clean the surface; accord...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com