Mineralized exenatide release system and preparation method and application thereof
A technology of exenatide and system, which is applied in the field of novel exenatide sustained-release preparations, can solve the problems of immune rejection in the body, easy to induce matrix immune response, and influence on the biological activity of exenatide.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of Mineralized Exenatide
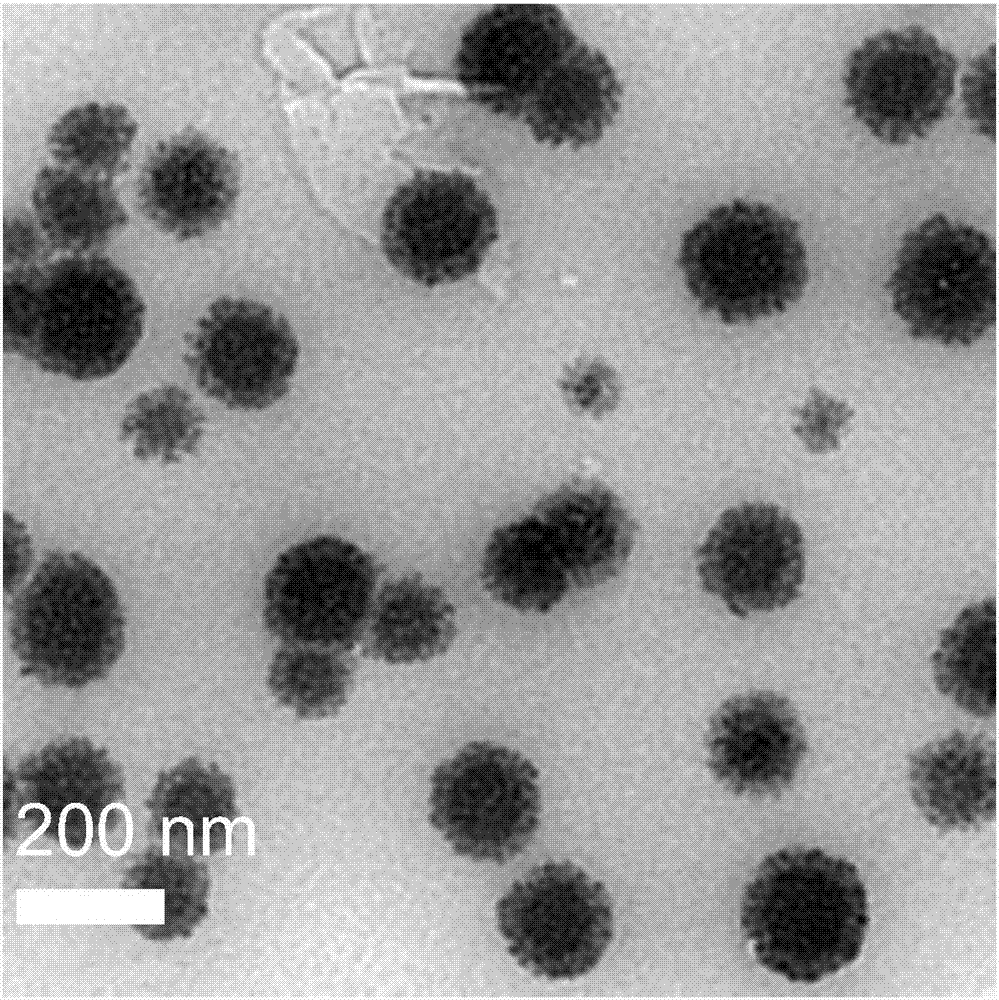
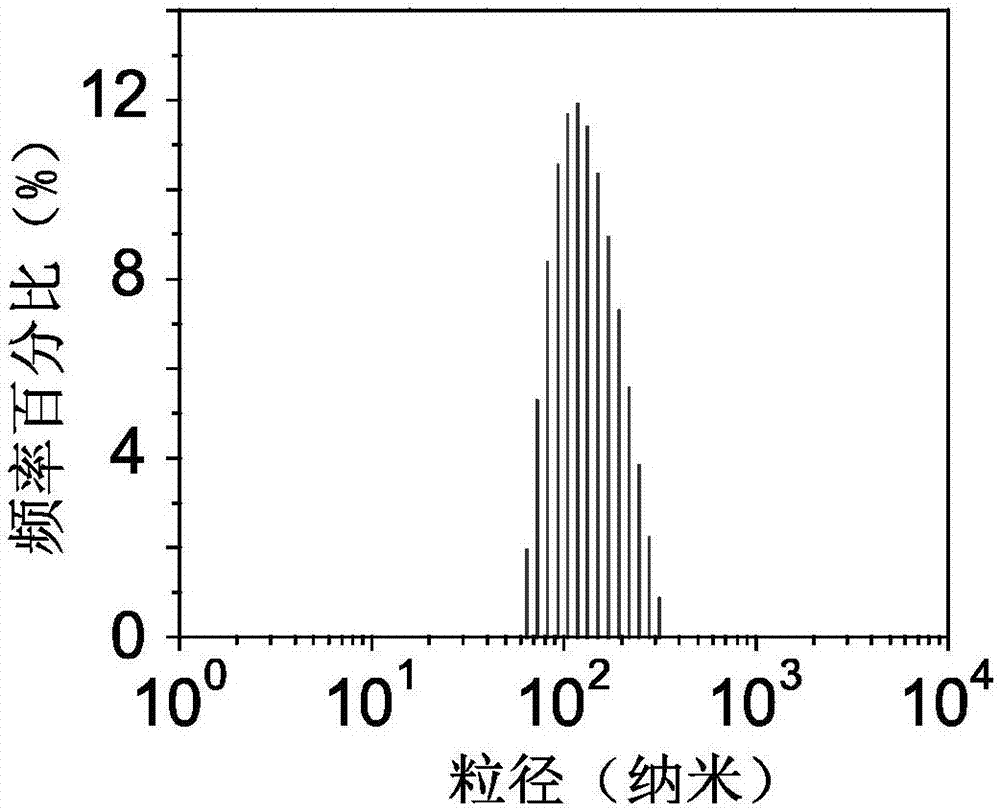
[0036] Weigh 2 mg of exenatide and dissolve it in 1 ml of DMEM cell culture medium, and place the solution at 37 degrees Celsius for 24 hours. Weigh 110 mg of calcium chloride solid and dissolve it in 1 ml of ultrapure water to obtain a 1 mol / L calcium chloride solution. Add 10 microliters of calcium chloride solution to 1 milliliter of exenatide DMEM solution, and react at 37 degrees Celsius in a 5% carbon dioxide environment for 24 hours. The obtained solution was centrifuged by ultrafiltration, the molecular weight cut-off was 100k, the centrifugation speed was 6000 rpm, and the centrifugation time was 20 minutes. Obtained mineralized exenatide utilizes the transmission electron microscope to observe the morphology, and it can be known that the mineralized exenatide is a nanoparticle system composed of exenatide at the center and its surrounding biomimetic mineral calcium phosphate (such as figure 1 shown), using a ...
Embodiment 2
[0037] Example 2: In Vitro Release of Exenatide
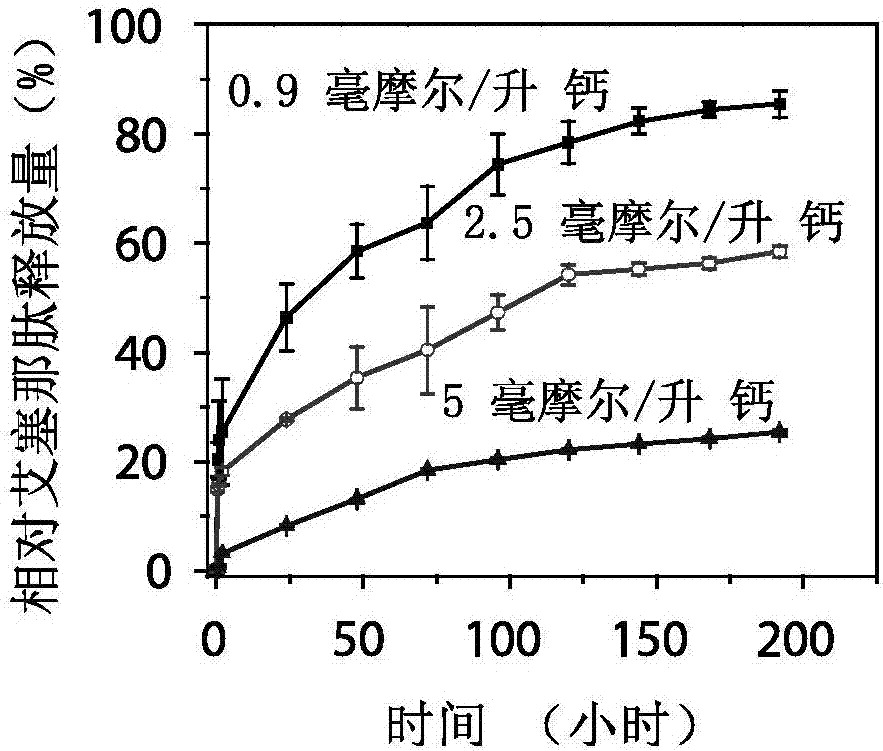
[0038] The mineralized exenatide particles prepared in Example 1 were dispersed in 1 ml of DMEM solutions containing different calcium ion concentrations (0.9, 2.5 or 5 mmol / L). At the time points of 0, 0.5, 1, 2, 24, 48, 72, 96, 120, 144, 168, and 192 hours, the reaction system was centrifuged by ultrafiltration (100k molecular weight cut-off, 6000 rpm, 20 minutes), The remaining solid was separated from the supernatant. The drug concentration in the supernatant was determined using a commercial exenatide ELISA kit. Finally, the residual particles were reacted in the pH 5 solution environment for 24 hours to dissolve the particles completely, measure the maximum release amount, set it as 100%, and calculate the relative release amount at each time point (such as image 3 shown). The structure of the released exenatide was determined by circular dichroism chromatography and compared with exenatide without any treatment (eg F...
Embodiment 3
[0039] Example 3: In vivo release of exenatide
[0040] Cysteine was connected to position 39 of exenatide to form a new reactive site (side chain of -SH group), which was labeled with near-infrared fluorescent dye IRdye 800CW. The specific operation is as follows: first, N-hydroxysuccinimide-modified IRdye 800CW (25 microliters, 10 mmol / liter) was added to 500 microliters of dimethyl sulfoxide, and then 20 microliters of N-( 2-Aminoethyl) maleimide trifluoroacetate (3 mg / ml dimethyl sulfoxide solution), stirred for 4 hours. Then, 1 mg of exenatide with one cysteine inserted at the C-terminus was added to the above reaction system and reacted overnight (about 12 hours). The resulting product was separated using high performance liquid chromatography, and its structure was characterized using Funky. Thereafter, the near-infrared fluorescent dye-labeled exenatide and its mineralized particles were injected subcutaneously, and the fluorescent signal at the injection point w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com