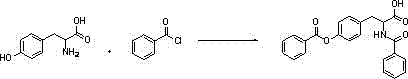Novel method for synthesizing tiropramide hydrochloride
A technology of tiromide hydrochloride and a new method, which is applied in the field of synthesizing tiromide hydrochloride, can solve the problems of high cost, low yield, and low purity, and achieve the effects of simple and mild reaction, improved economic benefits, and simplified post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Step 1: In a 5000ml four-necked reaction flask, add 2000ml of purified water, 110g of sodium carbonate, control the temperature to 10°C, add 100g of L-tyrosine, 100g of benzoyl chloride, control the temperature to 25°C, stir for 30min, drop Methanol 600ml, stirred for 1h, cooled to 5°C, and concentrated hydrochloric acid 700g was added dropwise. Stir for 1 h, centrifuge, wash with 300 ml of purified water, and dry the solid in vacuum at 80°C for 10 hours to obtain 210 g of dry O,N-2-benzoyl-L-tyrosine (content 99%).
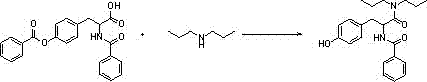
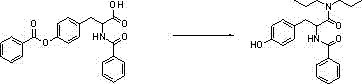
[0029] Step 2: Add 1500ml of toluene into a 3000ml four-necked bottle, put in 100g of O,N-2-benzoyl-L-tyrosine and 90g of dipropylamine, heat up to reflux and divide water, react for 10h, and add the prepared concentrated hydrochloric acid 23g of dilute hydrochloric acid and 300g of purified water, stirred for 30min, separated into layers, and removed the water layer. Add 90g of sodium chloride and 300ml of purified water, stir for 30min, separate layers,...
Embodiment 2
[0032] Step 1: In a 5000ml four-necked reaction flask, add 2000ml of purified water, 143g of potassium carbonate, control the temperature to 10°C, add 100g of L-tyrosine, 100g of benzoyl chloride, control the temperature to 25°C, stir for 30min, drop Methanol 600ml, stirred for 1h, cooled to 5°C, and concentrated hydrochloric acid 700g was added dropwise. Stir for 1 h, centrifuge, wash with 300 ml of purified water, and dry the solid in vacuum at 80°C for 10 hours to obtain 203 g of dry O,N-2-benzoyl-L-tyrosine (content 99%).
[0033] Step 2: Add 1500ml of chlorobenzene into a 3000ml four-necked bottle, put in 100g of O,N-2-benzoyl-L-tyrosine and 90g of dipropylamine, heat up to reflux and divide water, react for 10h, add the prepared concentrated Dilute hydrochloric acid with 23 g of hydrochloric acid and 300 g of purified water was stirred for 30 min, separated into layers, and the water layer was removed. Add 90g of sodium chloride and 300ml of purified water, stir for 30m...
Embodiment 3
[0036] Step 1: In a 5000ml four-necked reaction flask, add 2000ml of purified water, 150g of sodium carbonate, control the temperature to 20°C, add 100g of L-tyrosine, 110g of benzoyl chloride, control the temperature to 30°C, stir for 30min, drop Methanol 600ml, stirred for 1h, cooled to 5°C, and concentrated hydrochloric acid 700g was added dropwise. Stir for 1 h, centrifuge, wash with 300 ml of purified water, and dry the solid in vacuum at 80°C for 10 hours to obtain 206 g of dry O,N-2-benzoyl-L-tyrosine (content 99%).
[0037] Step 2: Add 1500ml of toluene into a 3000ml four-necked bottle, put in 100g of O,N-2-benzoyl-L-tyrosine and 120g of dipropylamine, raise the temperature to reflux and divide water, react for 10 hours, and add the prepared concentrated hydrochloric acid 25g of dilute hydrochloric acid and 300g of purified water, stirred for 30min, separated into layers, and removed the water layer. Add 90g of sodium chloride and 300ml of purified water, stir for 30m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com