Progesterone slow-release nanoparticles, preparation method of progesterone slow-release nanoparticles and progesterone slow-release injection
A technology of progesterone and nanoparticles, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Oily progesterone has long half-life and other problems, and achieves the effect of maintaining effective blood drug concentration, good patient compliance, high drug loading and encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The present invention also provides a preparation method of progesterone sustained-release nanoparticles, comprising the following steps:
[0048] 1) dissolving the progesterone and the polymer in an organic solvent to form an organic phase;
[0049] 2) Pour the organic phase obtained in step 1) into an aqueous solution containing an emulsifier, and undergo high-speed (6000rpm-10000rpm) shear emulsification to obtain an oil-in-water primary emulsion; subject the oil-in-water primary emulsion to high pressure ( 600bar~800bar) homogenize to obtain the final emulsion;
[0050] 3) After diluting the final emulsion obtained in step 2) with water, solidify at low temperature (0-5° C.) to obtain a suspension of particles;
[0051] 4) After the microparticle suspension obtained in step 3) is removed by tangential flow to remove the organic solvent and free drug, the progesterone sustained-release nanoparticles are obtained.
[0052] In order to achieve the above purpose, the ...
Embodiment 13
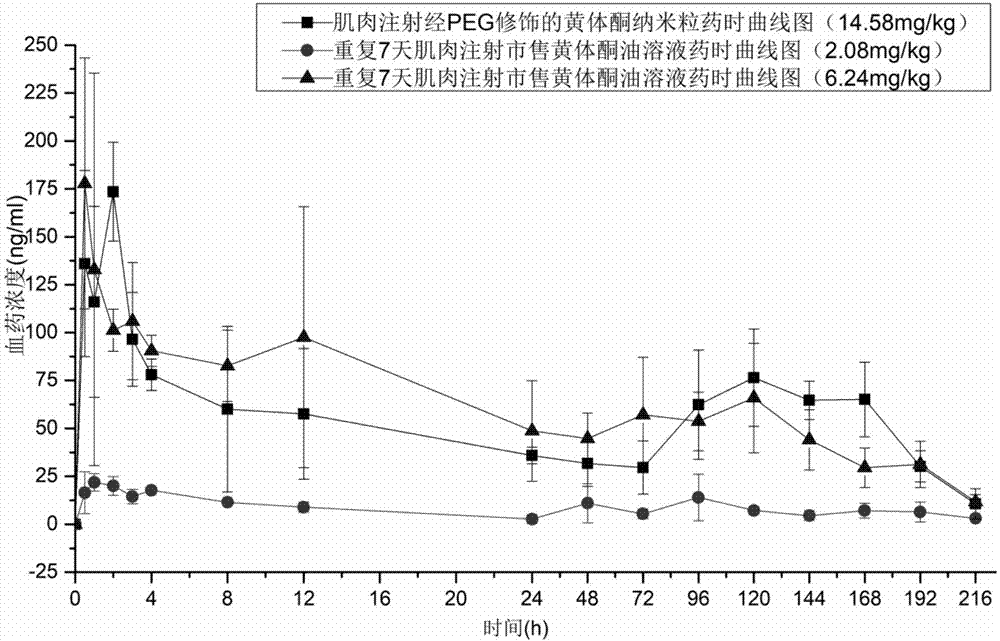
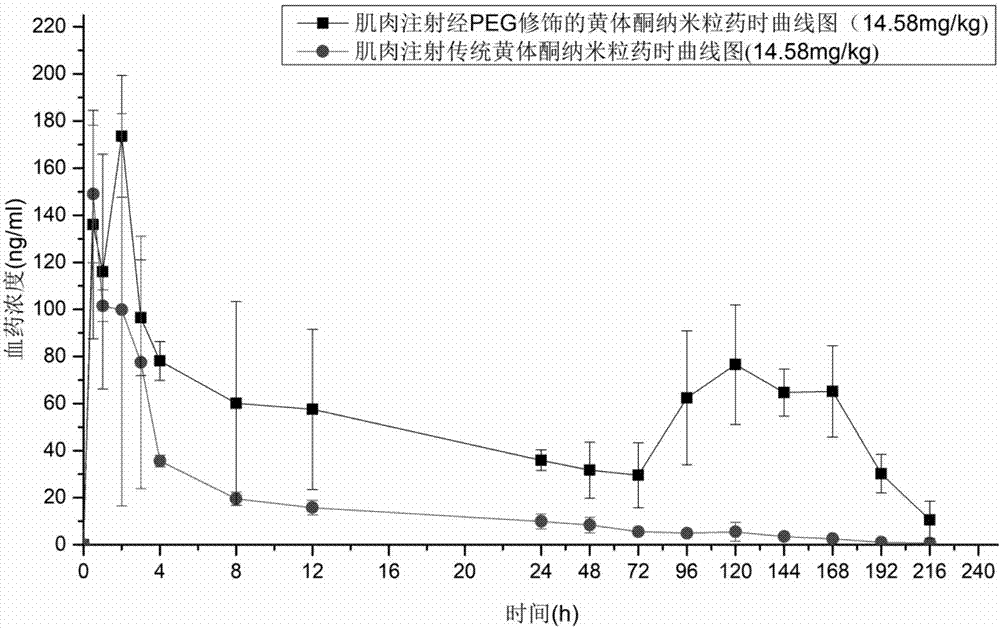
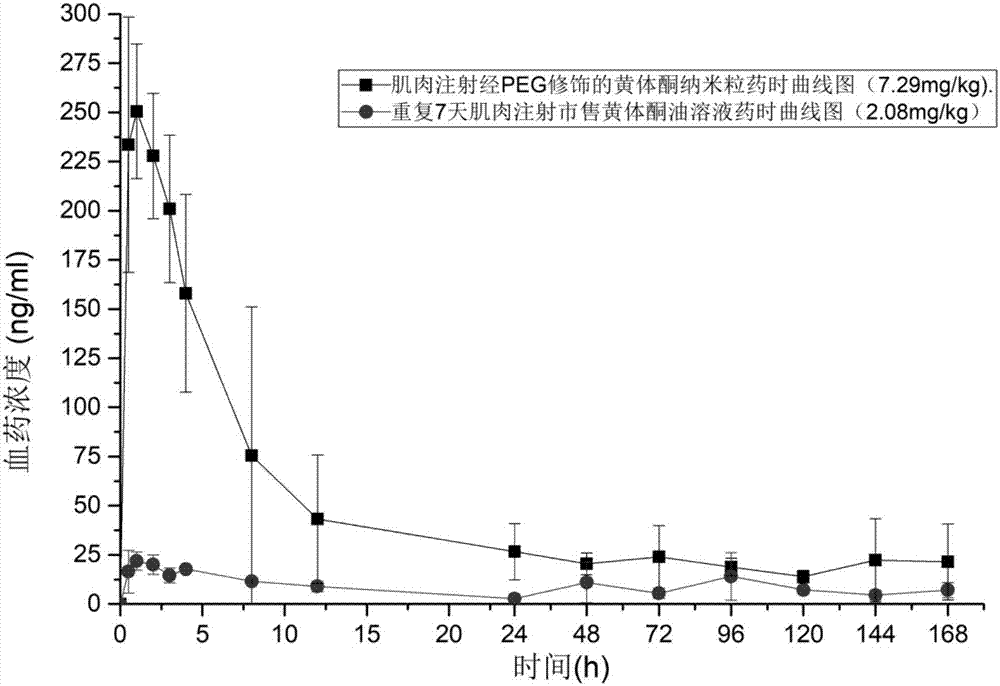
[0098] The pharmacokinetic analysis of embodiment 13 progesterone long-acting preparation of the present invention
[0099] The progesterone nanoparticles of 2.08mg (calculated as progesterone) prepared according to the method of Example 2 are intramuscularly injected into the hind leg biceps femoris of SD rats (about 200g of body weight), and commercially available oil solution and The common progesterone nanoparticles prepared in Comparative Example 1 were used as a control test. The solvent for injection in the sustained-release preparation of the present invention is the nanoparticle reconstitution agent for injection, and its composition is 0.9% sodium chloride water for injection.
[0100] Blood was collected from the venous plexus behind the eyes of the rats regularly, and the drug concentration of progesterone in plasma was determined by establishing an LC-MS method. See the blood concentration of administration 0-196h figure 1 , figure 1 It is the concentration cur...
Embodiment 14
[0116] Embodiment 14 progesterone long-acting preparation characteristic test
[0117] external assessment. The progesterone nanoparticles prepared in the above-mentioned comparative example 1 and embodiment 2 were observed under a transmission electron microscope, and the results can be found in Figure 5 and Figure 6 . Figure 5 The transmission electron microscope image (TEM) of the PEG-modified progesterone nanoparticles prepared for the embodiments of the present invention, Figure 6 Transmission electron micrograph (TEM) of traditional progesterone nanoparticles prepared for Comparative Example 1 of the present invention. It can be seen from the above results that the nanoparticles prepared in Example 2 of the present invention and Comparative Example 1 are regular and circular, and the particle diameters are equivalent.
[0118] Drug loading: Precisely measure 10 mg of the nanoparticles prepared in the above examples, put them in a 25 mL volumetric flask, add 1 mL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com