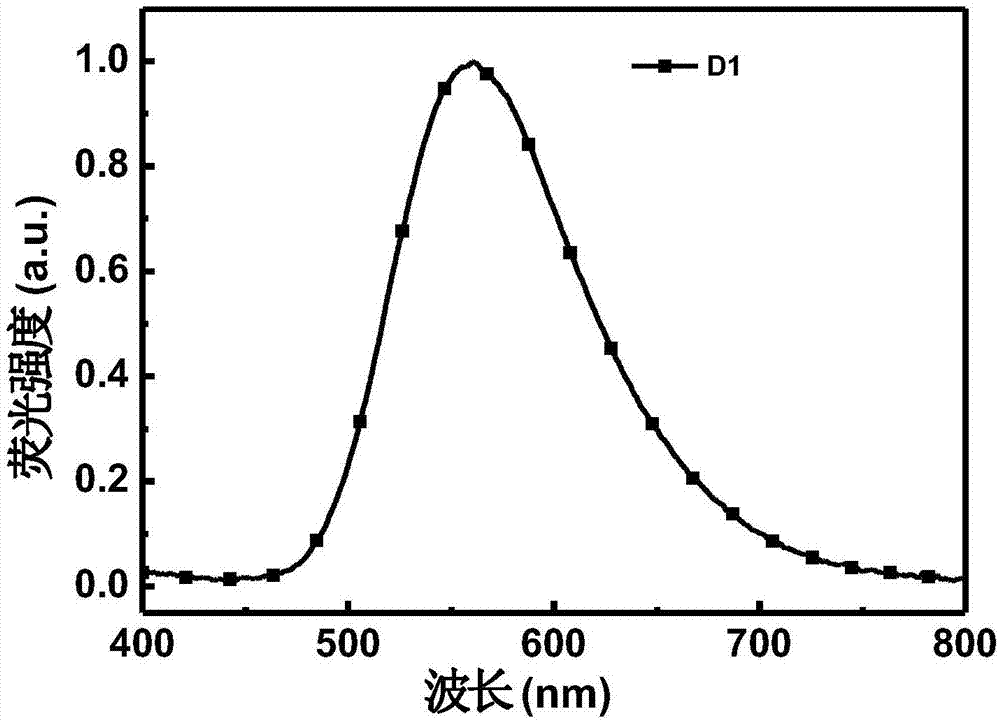
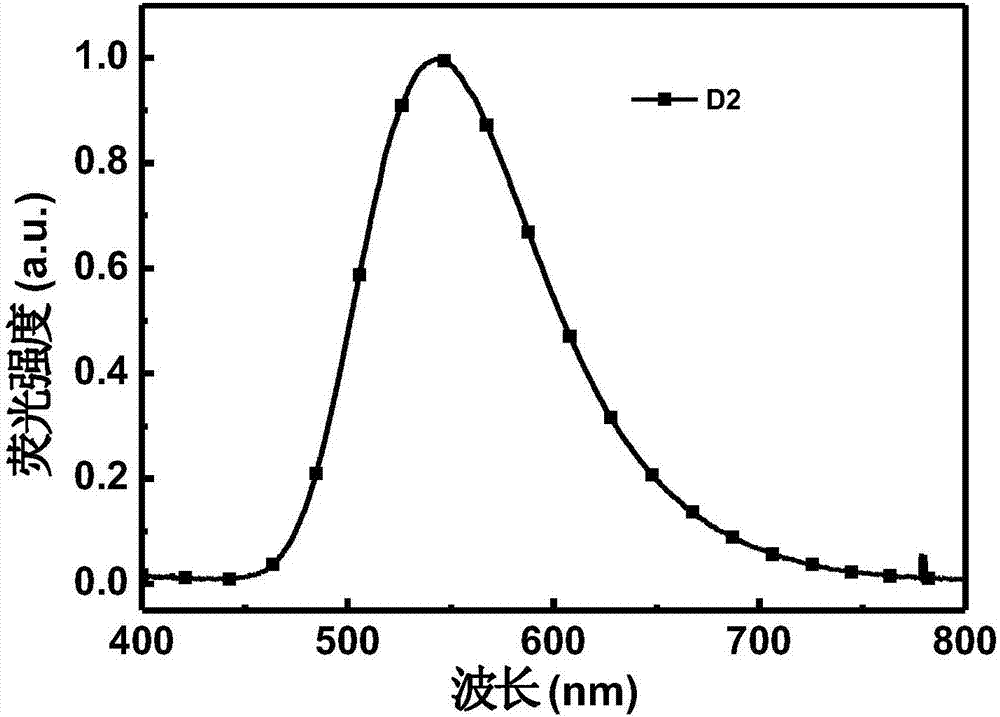
Fluorescent material containing beta-diketone structure as well as preparation method and application thereof
A fluorescent material and diketone technology, applied in the field of fluorescent materials and OLED devices, can solve the problems of poor effect and low solid-state quantum yield, and achieve the effect of overcoming short fluorescence lifetime, high solid-state quantum yield, and long fluorescence lifetime
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The preparation of 1,3-bis(4-(10H-phenoxazin-10-yl)phenyl)-3-hydroxyprop-2-en-1-one, the reaction process is as follows:
[0060]
[0061] 1.3 g of 1,3-bis(4-bromophenyl)-3-hydroxyprop-2-en-1-one, 1.3 g of phenoxazine, 68 mg of palladium acetate, 270 mg of tri-tert-butylphosphinetetrafluoroboron Acid salt, 670 mg of sodium tert-butoxide and 50 ml of redistilled toluene were added to a 100 ml single-necked round-bottom flask, refluxed for 48 hours under the protection of argon, cooled to room temperature, quenched with saturated brine, extracted with dichloromethane, The organic phase was dried over anhydrous sodium sulfate, filtered and spin-dried. The product was passed through the column at a volume ratio of n-hexane: dichloromethane 1:1 to obtain 1.5 g of the product. Brown-red solid, yield 72%.
[0062] Obtained molecular weight: 586.4.
[0063] Elemental analysis results: C 79.95%, H 4.48%, N 4.78%.
Embodiment 2
[0065] The preparation of 1-(4-(10H-phenoxazin-10-yl)phenyl)-3-hydroxy-3-phenylprop-2-en-1-one, the reaction process is as follows:
[0066]
[0067] 2.1 g of 1-(4-bromophenyl)-3-hydroxy-3-phenylprop-2-en-1-one, 1.3 g of phenoxazine, 68 mg of palladium acetate, 270 mg of tri-tert-butylphosphine tetrafluoro Add borate, 670 mg of sodium tert-butoxide and 50 ml of redistilled toluene into a 100 ml single-necked round-bottom flask, reflux for 48 hours under the protection of argon, cool to room temperature, quench with saturated saline, and extract with dichloromethane , dried the organic phase with anhydrous sodium sulfate, filtered, and spin-dried. 2.3 g of the product was obtained by passing through the column with n-hexane:dichloromethane at a volume ratio of 1:1. Brown-red solid, yield 81%.
[0068] The molecular weight obtained by mass spectrometry: 405.3.
[0069] Elemental analysis results: C 79.99%, H 4.75%, N 3.45%.
Embodiment 3
[0071] The preparation of 1,3-bis(4-(9,9-dimethylacridin-10(9H)-yl)phenyl)-3-hydroxyprop-2-en-1-one, the reaction process is as follows:
[0072]
[0073] 1.3 g of 1,3-bis(4-bromophenyl)-3-hydroxyprop-2-en-1-one, 1.5 g of 9,9-dimethylacridine, 68 mg of palladium acetate, 270 mg of tritert Add butylphosphine tetrafluoroborate, 670 mg sodium tert-butoxide and 50 ml redistilled toluene into a 100 ml single-necked round bottom flask, reflux for 48 hours under argon protection, cool to room temperature, and quench with saturated saline , extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered, and spin-dried. The product was passed through the column at a volume ratio of n-hexane: dichloromethane of 1:1 to obtain 1.7 g of the product. Yellow solid, yield 78%.
[0074] Obtained molecular weight: 638.4.
[0075] Elemental analysis results: C 84.75%, H 6.08%, N 4.46%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com