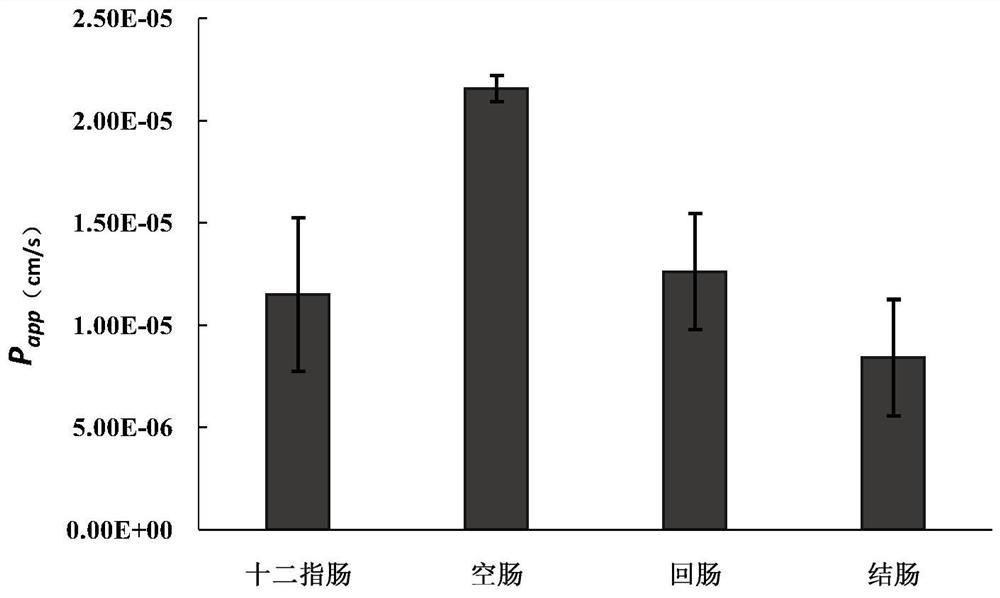
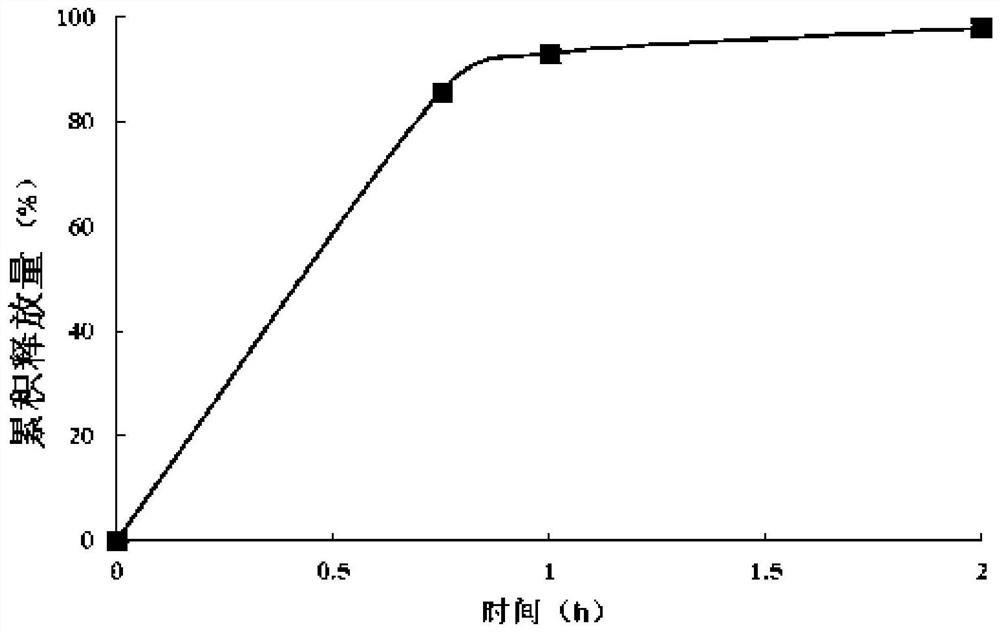
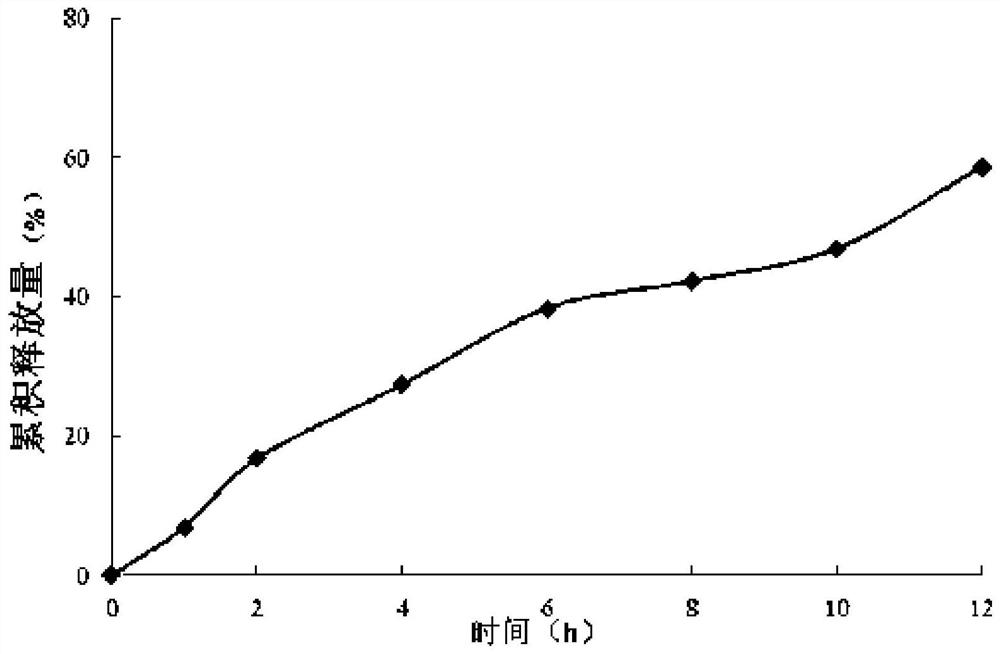
A kind of sustained-release dry suspension of mosapride and preparation method thereof
The technology of dry suspension and mosapride citrate, which is applied in the field of slow-release dry suspension of mosapride and its preparation, can solve the problems of complicated process, reduced production efficiency, low release degree and the like, To achieve the effect of simple preparation process, high production efficiency and improved taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] a. Prescription and preparation method of mosapride citrate immediate-release granules
[0062] components Weight (g) Mosapride citrate composition 150 lactose 150
[0063] Preparation Process:
[0064] Put mosapride citrate and sodium carboxymethyl starch in a ratio of 1:2 into a pulverizer, pulverize for 20 minutes, collect the composition, pass through an 80-mesh sieve, and set aside. Mix the co-pulverized composition obtained above with the prescribed amount of lactose, use 50% (w / w) ethanol solution as a binder to make a soft material, and adopt wet granulation or extrusion spheronization to granulate. The wet granules were dried in a fluidized bed for 30 minutes, and the dry granules with a particle size between 40 mesh and 60 mesh sieve were collected to obtain mosapride citrate immediate-release granules.
[0065] b. Prescription and preparation method of mosapride citrate sustained-release granules
[0066] components ...
Embodiment 2
[0076] a. Prescription and preparation method of mosapride citrate immediate-release granules
[0077] components Weight (g) Mosapride citrate composition 150 microcrystalline cellulose 130
[0078] Preparation Process:
[0079] The preparation method of the composition of mosapride citrate and sodium carboxymethyl starch is the same as embodiment one. Take the prescribed amount of mosapride citrate composition and mix with microcrystalline cellulose, use 5% (w / w) povidone K30 aqueous solution as the binder to make soft material, adopt wet granulation or extrusion spheronization . The wet granules were dried in a fluidized bed for 30 minutes, sieved and sized, and the dry granules with a particle size between 40 mesh and 60 mesh were collected to obtain the mosapride citrate immediate-release granules.
[0080] b. Prescription and preparation method of mosapride citrate sustained-release granules
[0081] components Weight (g) M...
Embodiment 3
[0093] a. Prescription and preparation method of mosapride citrate immediate-release granules
[0094] components Weight (g) Mosapride citrate composition 150 starch 160
[0095] Preparation Process:
[0096] The preparation method of the composition of mosapride citrate and sodium carboxymethyl starch is the same as embodiment one. Take the prescribed amount of mosapride citrate composition and mix it with starch, use water as a binder to make a soft material, and adopt wet granulation or extrusion spheronization to granulate. The wet granules were dried in a fluidized bed for 30 minutes, sieved and sized, and the dry granules with a particle size between 40 mesh and 60 mesh were collected to obtain the mosapride citrate immediate-release granules.
[0097] b. Prescription and preparation method of mosapride citrate sustained-release granules
[0098] components Weight (g) Mosapride citrate composition 300 starch 160 s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com