A method for detecting the specific immunoglobulin e of Penaeus lanceolata
An immunoglobulin and detection method technology, which is applied in the field of detection of specific immunoglobulin of Penaeus knives, can solve the problems of high detection cost, interference of allergy detection results, and inability to standardize the sensitization efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of fluorescent probes with different particle sizes FHMNs@PDDA@PAA
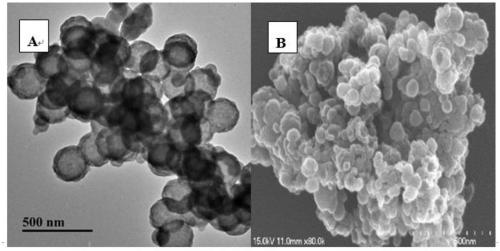
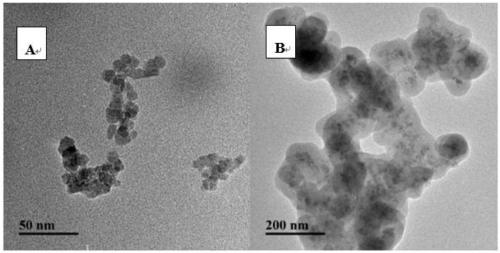
[0032] Dissolve cetyltrimethylammonium bromide (CTAB) powder in a mixture of 29mL deionized water, 12mL ethanol and 1mL ammonia water, then add polystyrene microsphere dispersion (dispersed in 10ml deionized ionized water), after magnetically stirring at room temperature for 60 min, tetraethyl orthosilicate (TEOS) was added dropwise, maintaining a constant stirring speed of 150 rpm / min, and continuing to stir at room temperature for 48 h. After the reaction was completed, the reaction solution was vacuum filtered and washed several times, and then dried in a vacuum oven at 65°C for 12 hours to obtain core-shell PS / SiO 2 Composite microsphere powder. Calcined at 550°C for 8h to remove template polystyrene to obtain hollow silica. Disperse 0.05g of HMNs and 0.012g of 5(6)-fluorescein isothiocyanate (FITC) in 5ml of deionized water, stir at room temperature for 48h, centrifuge the...
Embodiment 2
[0034] Example 2: Magnetic probe Fe 3 o 4 @SiO 2 Preparation of @PAA Nanoparticles
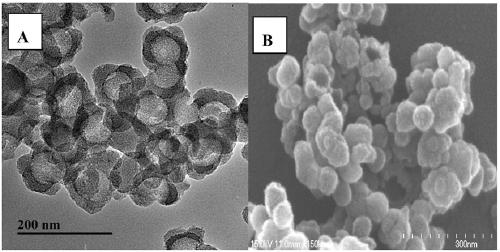
[0035] Weigh 300mgFe 3 o 4 Disperse in a mixture of 40mL ethanol and 4mL deionized water, after ultrasonication for 15min, maintain a certain stirring speed, add 5mL ammonia water, slowly add 2mLTEOS, react at room temperature for 12h, and use 0.1moL / L hydrochloric acid solution and deionized water after magnetic separation Wash each for 3 times, and dry at 40° C. for 12 hours to obtain a reddish-brown precipitate, which is ferric oxide / silicon dioxide core-shell nanoparticles. Fe will be produced 3 o 4 @SiO 2 Dispersed in dimethylamide (DMF), mixed with 33ml DMF dissolved in 2g polyacrylic acid (PAA), ultrasonicated for 30min, the mixture was vigorously stirred and heated to 110°C, added 3.3mL DMF dissolved in 4-dimethylaminopyridine and Dissolve N,N'-dicyclohexylcarbodiimide (DCC) in 6.6mL DMF, keep the mixture at 110°C and stir for 12h, then magnetically separate the product, wash w...
Embodiment 3
[0037] Example 3 pH optimization process, time optimization process, and temperature optimization process respectively provide pH optimization
[0038] Take appropriate amount of Fe 3 o 4 @SiO 2 @PAA—antigens and FHMNs@PDDA@PAA—Ab2 were added with positive IgE (specific immunoglobulin E) of Penaeus lanceolata, and after incubation at a certain temperature, the precipitate was collected by magnetic separation and washed with phosphate buffer (pH7.4) Wash 3 times, collect the precipitate by magnetic separation, add 3mL sodium hydroxide solution of different pH and sonicate for 20min, dissolve the hollow mesoporous shell, release fluorescent molecules, and detect the fluorescence intensity, the results are as follows: Figure 4 As shown in A, the fluorescence intensity is the highest at pH 11, so the sodium hydroxide solution at pH 11 is selected as the detection solution.
[0039] temperature optimization
[0040] Take appropriate amount of Fe 3 o 4 @SiO 2 @PAA—antigens a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com