Tyramine/diphosphonate hyaluronic acid macromolecular compound and hydrogel, and preparation method and application thereof
A polymer compound and bisphosphonate technology, applied in the field of bone repair materials, can solve the problems of weak tissue penetration and difficult bone repair materials to trace in vivo, and achieve the promotion of bone repair ability, good in vivo degradation characteristics, and good bone repair. The effect of repair ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
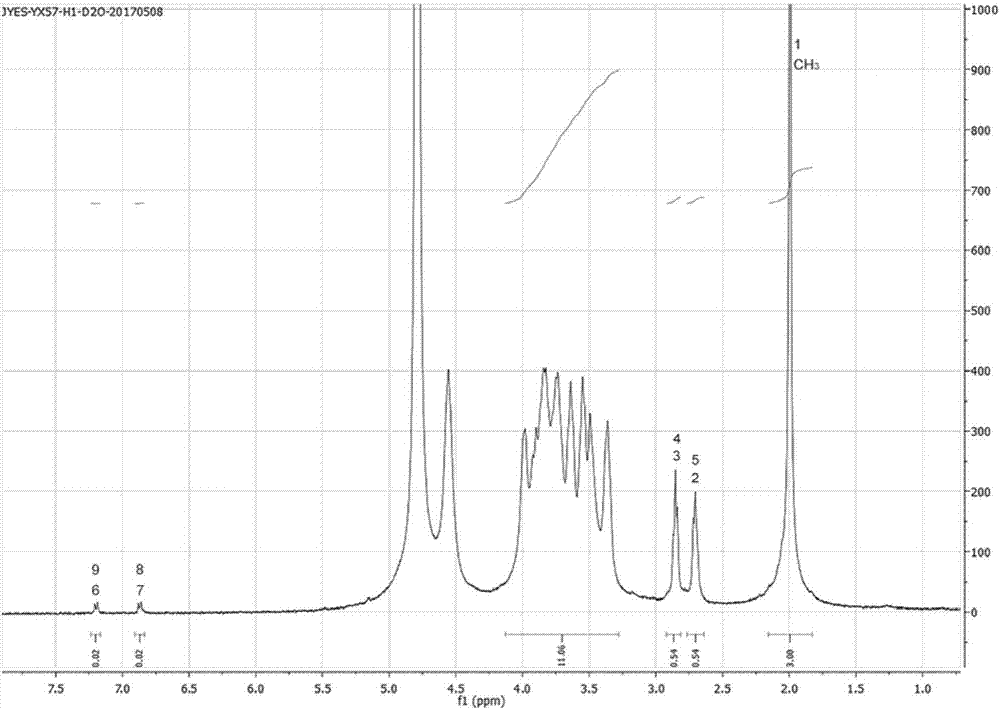
[0052] In this example, the preparation method of tyramine / bisphosphonate-hyaluronic acid polymer compound is provided, the steps are as follows:
[0053] (1) Dissolve 200 mg of hyaluronic acid in 25 mL of pure water, add 16.66 mg of functionalized linker small molecule 3,3'-dithiobis(propionic hydrazide) (3,3'-Dithiobis(propionic hydrazide)) and Stir and mix well, then add the tyramine solution formed by dissolving 41.15mg tyramine in 1mL dimethyl sulfoxide (DMSO) and stir well, then add 76.5mg 1-hydroxybenzotriazole (HoBt) dissolved in 1mL HoBt solution formed in DMSO and stirred;
[0054] (2) Adjust the pH value of the mixed solution obtained in step (1) to 4.7 with 1mol / L hydrochloric acid, and then add 71.85 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), stirred and reacted at room temperature for 16h;
[0055] (3) Regulate the pH value of the reaction solution obtained in step (2) to 8.7 with 1mol / L hydrochloric acid, then add 53.9mg dithiothr...
Embodiment 2
[0060] In this example, provide 131 The preparation method of the I-labeled tyramide / bisphosphonate-hyaluronic acid macromolecular compound is as follows:
[0061] Put 1mL of 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen) solution into a 5mL plastic centrifuge tube, and then use a vacuum pump for 20min to form a lodogen coating on the bottom of the centrifuge tube .
[0062] The tyramine / bisphosphonate-hyaluronic acid macromolecular compound prepared in Example 1 was prepared into a 2 mg / mL solution with pH=7.4 and a concentration of 10 mmol / L PBS buffer solution, which was referred to as solution A, and Na 131 I use pH = 7.4, PBS buffer solution with a concentration of 10mmol / L to prepare a 10mCi / mL solution, which is referred to as solution B. Add 1mL of solution A to the centrifuge tube containing the lodogen coating, then add 1mCi of solution B, and then add Ultrapure water to 2mL, shake the reaction for 15min and then purify the reaction product by ethanol prec...
Embodiment 3
[0067] In this example, provide 99m The preparation method of the Tc-labeled tyramide / bisphosphonate-hyaluronic acid polymer compound is as follows:
[0068] The tyramine / bisphosphonate-hyaluronic acid macromolecular compound prepared in Example 1 was prepared into a 2 mg / mL solution with PBS buffer solution with a pH of 7.4 and a concentration of 10 mmol / L.
[0069] The compound shown in formula (I) is prepared into a solution of 10 mg / mL with physiological saline or PBS buffer, which is referred to as solution C. 99m The water-soluble salt of Tc is prepared into a solution with a concentration of 100mCi / mL with PBS buffer solution with a pH of 7.4 and a concentration of 10mmol / L, which is recorded as solution D. Take 1mL of solution C, and add 20μL of chlorinated chloride with a concentration of 1mg / mL to it. Stannous solution and 2mCi solution D, shaking reaction for 15min, the resulting crude product solution was purified with G25 Sephadex column, the preparation method o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com