Dapagliflozin preparation method
A technology of reaction and chlorobenzaldehyde, which is applied in the field of chemical drug preparation, can solve the problems of serious pollution and achieve the effects of improved purity, short synthetic route and easy process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
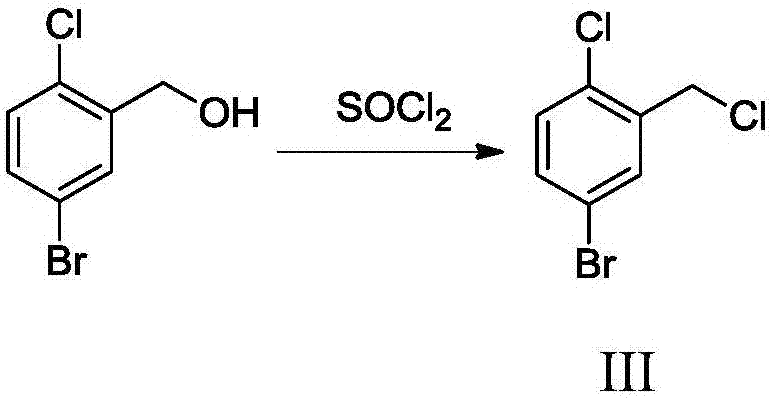
[0030] The preparation method of dapagliflozin of the present invention uses 2-chlorobenzaldehyde as a starting material, and synthesizes 5-bromo-2-chlorobenzyl chloride through bromination, reduction, and chlorination, and alkylates with phenetole through K. Reaction to synthesize 5-bromo-2-chloro-4'-ethoxydiphenylmethane, and then condense with 2,3,4,6-tetra-O-trimethylsilyl-D-gluconolactone, desorb Trimethylsilyl protection, etherification, and reductive demethoxylation to obtain the hypoglycemic drug dapagliflozin; specifically include the following steps:
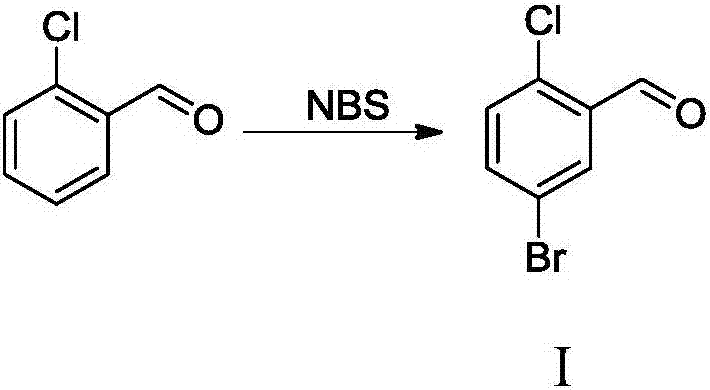
[0031] (1) Preparation of 5-bromo-2-chlorobenzaldehyde (I): 2-chlorobenzaldehyde and NBS are dissolved in a polar solvent, and react for 10-12h under the condition of <5°C to prepare 5-bromo- 2-Chlorobenzaldehyde (I); Wherein, the polar solvent can be selected from any one of dichloromethane, chloroform, carbon tetrachloride, dimethylformamide, tetrahydrofuran or acetonitrile, preferred toxicity and low boiling point d...
Embodiment 1
[0044] The preparation of embodiment 1 5-bromo-2-chlorobenzaldehyde (I)
[0045]Take 140kg of 2-chlorobenzaldehyde, dissolve it in 400kg of dichloromethane, stir in ice bath for 30min, then add 180kg of NBS in batches, keep the temperature of the reaction system below 5°C, after the addition, react for 10h, after the reaction, filter , The filtrate was washed with 300kg saturated aqueous sodium bicarbonate solution, then the organic layer was washed with water until neutral, dichloromethane was recovered, and the residual solid was recrystallized with petroleum ether: ethyl acetate (1:1) to obtain 212kg of white solid, with a yield of 98%.
Embodiment 2
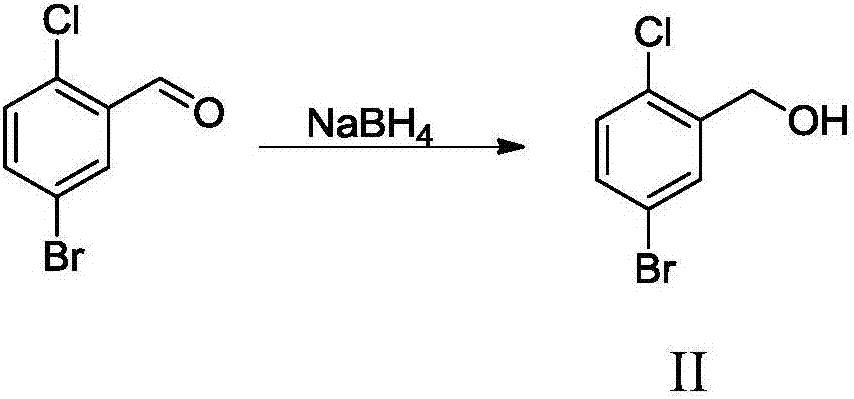
[0046] The preparation of embodiment 2 5-bromo-2-chlorobenzyl alcohol (II)
[0047] Take 108 kg of 5-bromo-2-chlorobenzaldehyde, dissolve it in 250 kg of absolute ethanol, add 25 kg of sodium borohydride, and react at room temperature for 4 hours. After the reaction, recover most of the absolute ethanol under reduced pressure, and add dilute hydrochloric acid dropwise under ice bath Quench the reaction system, bring the pH of the system to 4-5, extract with 300kg ethyl acetate, wash with water until neutral, dry over anhydrous sodium sulfate, filter, recover the solvent from the filtrate under reduced pressure, and recrystallize from petroleum ether: ethyl acetate (1:1) Obtain light yellow solid powder 107kg, yield 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com