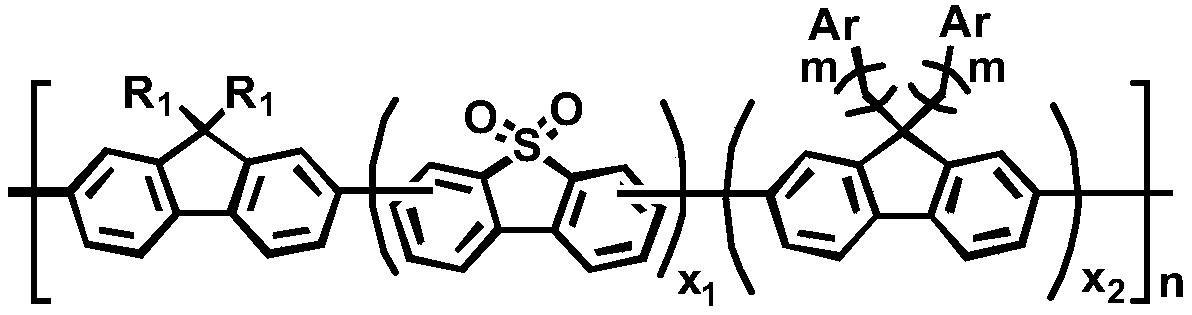
Poly(fluorene-co-s,s-dioxy-dibenzothiophene) derivatives containing anthracene derivatives in side chains, preparation method and application thereof
A technology of dibenzothiophene and anthracene derivatives is applied in the field of polyderivatives containing anthracene derivatives in side chains and their preparation fields, so as to achieve the effects of improving device stability, maintaining spectral purity and stability, and simplifying device preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of compound M1
[0040] Under nitrogen protection, 9,10-dibromoanthracene (3.36g, 10mmol), 1-naphthaleneboronic acid (1.72g, 10mmol), potassium carbonate (4.14g, 30mmol), tetrakis(triphenyl Phosphine) palladium (0.58 g, 0.5 mmol), 15 mL of distilled water and 100 mL of tetrahydrofuran were heated to 70 ° C for 12 hours. After the reaction was completed, tetrahydrofuran was distilled off under reduced pressure, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and after removing the organic phase solvent, the crude product was mixed with petroleum ether:dichloromethane=8:1 (v / v) The solvent was used as an eluent for column chromatography to obtain 2.53 g of off-white solid with a yield of 66% (mass spectrum: 382.0).
[0041] The chemical reaction equation is as follows:
[0042]
Embodiment 2
[0043] Embodiment 2: the preparation of compound M2
[0044] Under the protection of nitrogen, in a 150mL three-neck flask, M1 (1.92g, 5mmol) was dissolved in 50mL of anhydrous tetrahydrofuran, and the temperature was kept at 0°C for 20 minutes in an ice bath. Slowly add n-butyllithium solution (4mL, 8mmol) dropwise into the reaction flask, stir at 0°C for 1 hour after the dropwise addition, then inject 1,4-dibromobutane (2.16g, 10mmol) into the reaction flask , continue to react in an ice bath for 2 hours, and naturally rise to room temperature for 12 hours. Tetrahydrofuran was removed by distillation under reduced pressure, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and the organic phase solvent was removed by distillation under reduced pressure, then mixed with petroleum ether:dichloromethane=10:1 (v / v) The solvent was used as eluent and purified by column chromatography to obtain 1.54 g of off-white ...
Embodiment 3
[0047] Embodiment 3: the preparation of compound M3
[0048] Under nitrogen protection, add 2,7-dibromofluorene (1.30g, 4mmol), potassium hydroxide (1.68g, 30mmol) and 50mL tetrahydrofuran into a 150mL three-necked flask, stir at room temperature for 2 hours, then add M2 (4.39g , 10 mmol), heated to 70 ° C for 24 hours. After the reaction is complete, THF is removed by rotary evaporation, the product is extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and the organic phase solvent is removed by distillation under reduced pressure. ) mixed solvent as eluent and purified by column chromatography to obtain 2.41 g of light yellow-green solid with a yield of 58%, (mass spectrum: 1040.3).
[0049] The chemical reaction equation is as follows:
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com