Asymmetric alpha-diimine nickel (II) complex for polymerizing ethylene and 1-hexene with o-benzhydryl substituent
A technology of benzhydryl aniline and benzhydryl, which is applied in the field of asymmetric α-diimine nickel complexes, can solve the problems of easy loss of activity and poor stability, and achieve increased instability, high stability, Effect of reducing transfer rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of asymmetric α-diimine ligand:
[0037] Mix 4-methyl-2,6-benzhydrylaniline and excess diacetyl at a molar ratio of 1:1.5, use toluene as solvent, add 0.05 times 4-methyl-2,6-benzhydryl The p-toluenesulfonic acid (TsOH) of the molar amount of base aniline is used as a catalyst, and after reflux reaction overnight at 105° C., the solvent and excess diacetyl are rotary evaporated to obtain a crude product, which is then used in C 2 h 5 Washing with OH gave a yellow solid, ortho-benzhydryl substituted monoketone. The yield was 89%.
[0038] The above-mentioned ortho benzhydryl substituted monoketone and 2,6-diisopropylaniline are mixed in a molar ratio of 1:1.2, and toluene is used as a solvent, and p-toluenesulfonic acid ( TsOH) was used as a catalyst, and after reflux reaction at 105°C for 20 hours, the solvent was removed to obtain a crude product, which was then purified by C 2 h 5 OH / CH 2 Cl 2 The mixed solvent was recrystallized to precipitate ...
Embodiment 2
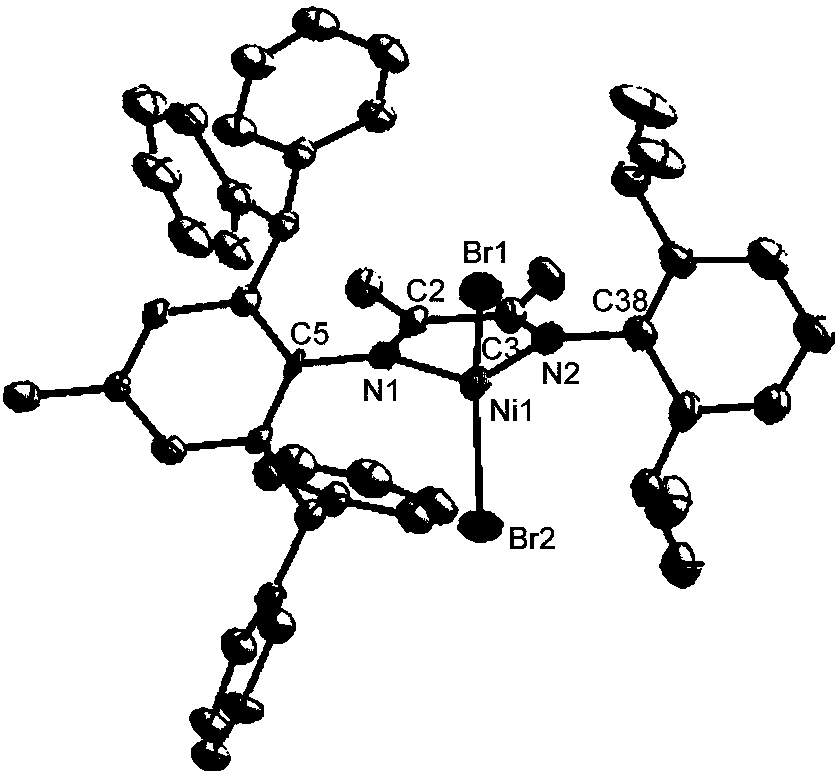
[0049] (1) Synthesis of the complex: under the protection of nitrogen, add the above-mentioned asymmetric ligand containing 2,6-dimethyl substitution (0.61g, 1.0mmol, the ligand refers to step 1 of Example 1) into a 100mL dry reaction tube (1) method synthesis), then add NiBr 2 (DME) (0.34g, 1.1mmol) and dichloromethane 20mL, stirred and reacted at room temperature for 12 hours, filtered the suspension, removed the solvent from the mother liquor under vacuum, washed the residue three times with ether (3×15mL), and dried in vacuo 0.71 g of brown solid powder was obtained. The yield was 95%.
[0050] Anal. Calcd for (C 45 h 42 Br 2 N 2 Ni): C, 65.17; H, 5.10; N, 3.38. Found: C, 65.49; H, 5.42; N, 3.41. MALDI-TOF-MS(m / z):calcd for C 45 h 42 BrN 2 Ni: 747.1885,found: 747.1255[M-Br] + .
[0051] Its reaction formula is as follows:
[0052]
[0053] (2) Ethylene polymerization: replace the 250mL polymerization bottle with a magnetic stirrer with vacuum-nitrogen circula...
Embodiment 3
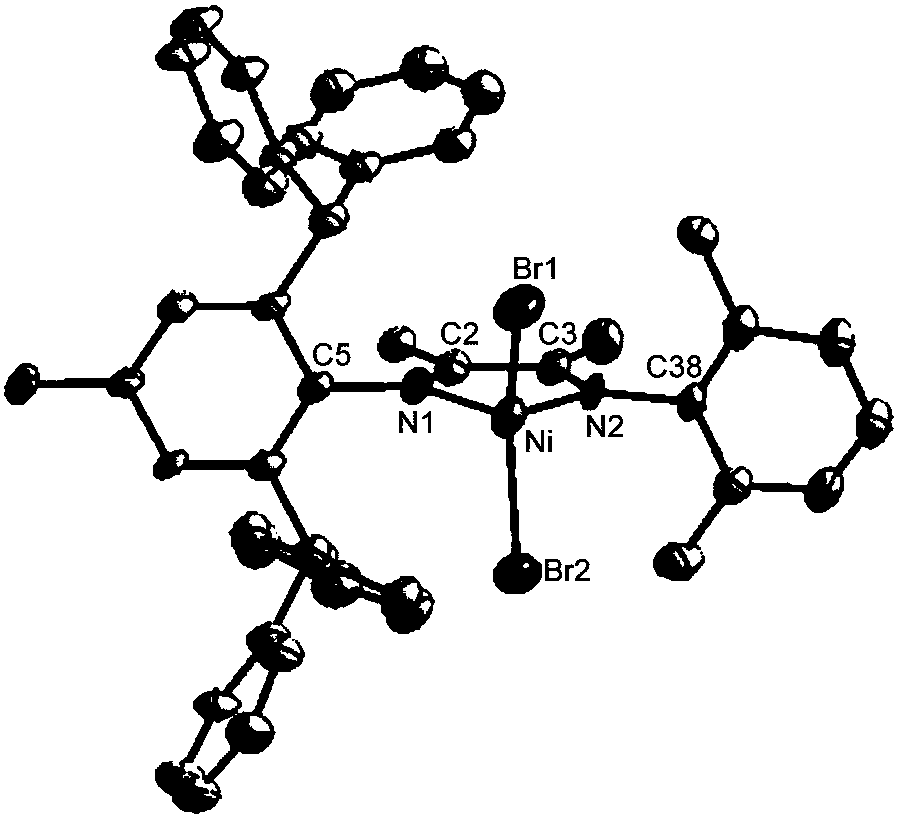
[0056] (1) Synthesis of the complex: under the protection of nitrogen, add the above-mentioned asymmetric ligand of aniline (0.58g, 1.0mmol, the ligand is synthesized according to the method of step (1) in Example 1) to a 100mL dry reaction tube, and then Join NiBr 2 (DME) (0.34g, 1.1mmol) and dichloromethane 20mL, stirred and reacted at room temperature for 12 hours, filtered the suspension, removed the solvent from the mother liquor under vacuum, and washed the residue three times with diethyl ether (3×17mL), dried in vacuo 0.66 g of brown solid powder was obtained. The yield was 92%.
[0057] Anal. Calcd for (C 43 h 38 Br 2 N 2 Ni): C, 64.45; H, 4.78; N, 3.50. Found: C, 64.49; H, 4.52; N, 3.45. MALDI-TOF-MS (m / z): calcd for C 43 h 38 BrN 2 Ni: 719.1572,found: 719.1244[M-Br] + .
[0058] Its reaction formula is as follows:
[0059]
[0060] (2) Ethylene polymerization: replace the 250mL polymerization bottle with a magnetic stirrer with vacuum-nitrogen circulat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Active | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com