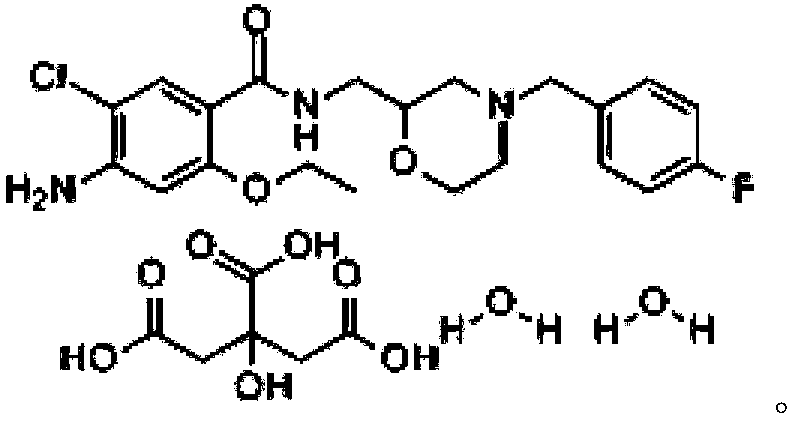
Mosapride citrate preparation and preparation method thereof
A mosapride citrate and lubricant technology, which is applied in the field of medicine, can solve the problems of complex preparation process, slow dissolution, poor stability, etc., and achieve the effects of good taste, increased absorption, and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Preparation Process:
[0032] (1) The preparation method of 12kg hydroxypropyl-β-cyclodextrin and 4kg mosapride citrate to form an inclusion compound: take 12kg hydroxypropyl-β-cyclodextrin and dissolve it in an appropriate amount of water to make saturated Aqueous solution, 4kg mosapride citrate is passed through 120 mesh sieves, is dissolved in the dehydrated ethanol solution of citric acid with an appropriate pH value of 6.0, under magnetic stirring, the mosapride citrate alcohol solution is slowly Slowly add the saturated aqueous solution of hydroxypropyl-β-cyclodextrin, continue to stir at room temperature for 2-4 hours after all the addition is complete, and remove most of the ethanol by rotary evaporation at 40-45°C. Put it into a shallow tray and put it in the freezer of the refrigerator. The pre-freezing time is 12 hours and the temperature is -50°C. When the temperature of the cold trap in the freeze-drying box reaches -50°C, put the sample in, vac...
Embodiment 2
[0035]
[0036]
[0037] Preparation Process:
[0038] (1) The preparation method of mosapride citrate inclusion compound is with embodiment 1.
[0039] (2) Mix the obtained clathrate, lactose, pregelatinized starch low-substituted hydroxypropyl cellulose evenly, add 2% hydroxypropyl cellulose and 50% ethanol solution to make a soft material, and use a 18-mesh sieve to make granules; The granules are dried at 50°C for 5 minutes; after the granules are dried, low-substituted hydroxypropyl cellulose and magnesium stearate are added and mixed, sieved with a 20-mesh sieve, and finally compressed into tablets.
Embodiment 3
[0041]
[0042] Preparation Process:
[0043] (1) The preparation method of mosapride citrate inclusion compound is with embodiment 1.
[0044] (2) Mix the obtained clathrate, lactose, pregelatinized starch, and low-substituted hydroxypropyl cellulose evenly, add 2% hydroxypropyl cellulose and 50% ethanol solution to make a soft material, and use a 18-mesh sieve to make granules; The granules are dried at 60°C for 20 minutes; after the granules are dried, low-substituted hydroxypropyl cellulose and magnesium stearate are added and mixed, sieved with a 20-mesh sieve, and finally compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com