Humanized anti-IgE monoclonal antibody
A monoclonal antibody and humanized technology, which is applied in the direction of antibodies, antibody medical components, chemical instruments and methods, etc., can solve the problems of poor thermal stability of anti-IgE antibodies, need to be refrigerated, and short shelf life, etc., to achieve long product shelf life, The effect of long storage time and high target antigen affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1, Preparation and screening of mouse-derived anti-IgE monoclonal antibody
[0037] Recombinant human IgE Fcε2- 4 protein (that is, the protein composed of the Cε2-Cε4 region of IgE), used to immunize Balb / c mice, intraperitoneally inject 50 μg / mouse / time, mix Freund's complete adjuvant for the first immunization, and mix Freund's non-adjuvant for 2-4 times Completely Adjuvanted. Two weeks later, the blood was taken from the orbit, and the antibody titer was detected by ELISA method. Animals with high titer were selected and injected intravenously with 50 μg per animal. Three days later, the spleen was taken, and the polyethylene glycol method was used for cell fusion, and the HAT medium (Invitrogen Company) was pressurized for screening. The supernatant of the fused cell culture was detected by ELISA to screen for positive clones, and cloned in time. Hybridoma cells were inoculated into the peritoneal cavity of Balb / c mice, ascites was regularly extracted, an...
Embodiment 2
[0043] Example 2. Cloning of antibody variable region genes (5'RACE)
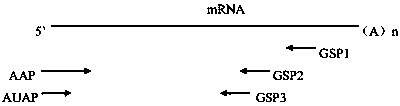
[0044] Trizol method (Trizol reagent was purchased from Thermofisher Company, product number 15596026) was used to extract the total RNA of the hybridoma cell No. 4F11 screened in Example 1. Select appropriate positions of antibody (IgG1, κ) heavy chain and light chain constant regions to design 3 gene-specific primers: GSP1-H~GSP3-H and GSP1-L~GSP3-L, the primer sequence is shown in SEQ ID NO:25 ~SEQ ID NO:30, wherein GSP1 is the farthest from the variable region gene and is used for reverse transcription reaction, GSP2 is used for the first round of PCR amplification, and GSP3 is used for nested amplification. AAP primers and AUAP primers in the 5' RACE cDNA end rapid amplification system (purchased from Thermofisher, Cat. No. 18374058) were used as universal primers. For the relative position of each primer on the PCR template, see image 3 .
[0045] The total RNA was reverse-transcribed into cDNA us...
Embodiment 3
[0046] Example 3, Humanized Design of Murine Monoclonal Antibody
[0047] The consensus sequence (SEQ ID NO: 18) of human immunoglobulin heavy chain subgroup III (SEQ ID NO: 17) and Igκ chain I subgroup (SEQ ID NO: 18) was selected as the humanized template of the antibody light and heavy chain, and then the heavy chain of the mouse antibody was The chain and light chain CDR regions were transplanted to the human template heavy chain III subgroup and Igκ chain I subgroup respectively, that is, replace the 24-34, 50-56 of SEQ ID NO:18 with CDR1, CDR2, and CDR3 of the light chain, respectively , 89-97 amino acids, respectively replacing amino acids 31-35, 49-66, and 99-102 of SEQ ID NO: 17 with CDR1, CDR2, and CDR3 of the heavy chain to form a CDR-grafted antibody, and its light chain and heavy chain The variable regions of have the sequences of SEQ ID NO: 19 and SEQ ID NO: 20, respectively.
[0048] Use the Insight II program package (purchased from Accelrys) to simulate the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com