Preparation and photocatalyst application of dibenzothiophene diketo conjugated microporous polymers
A benzodithiophenedione-based, conjugated microporous technology, which is applied in the preparation of organic compounds, catalytic reactions, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of general selectivity and achieve Wide application prospects, high conversion rate, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
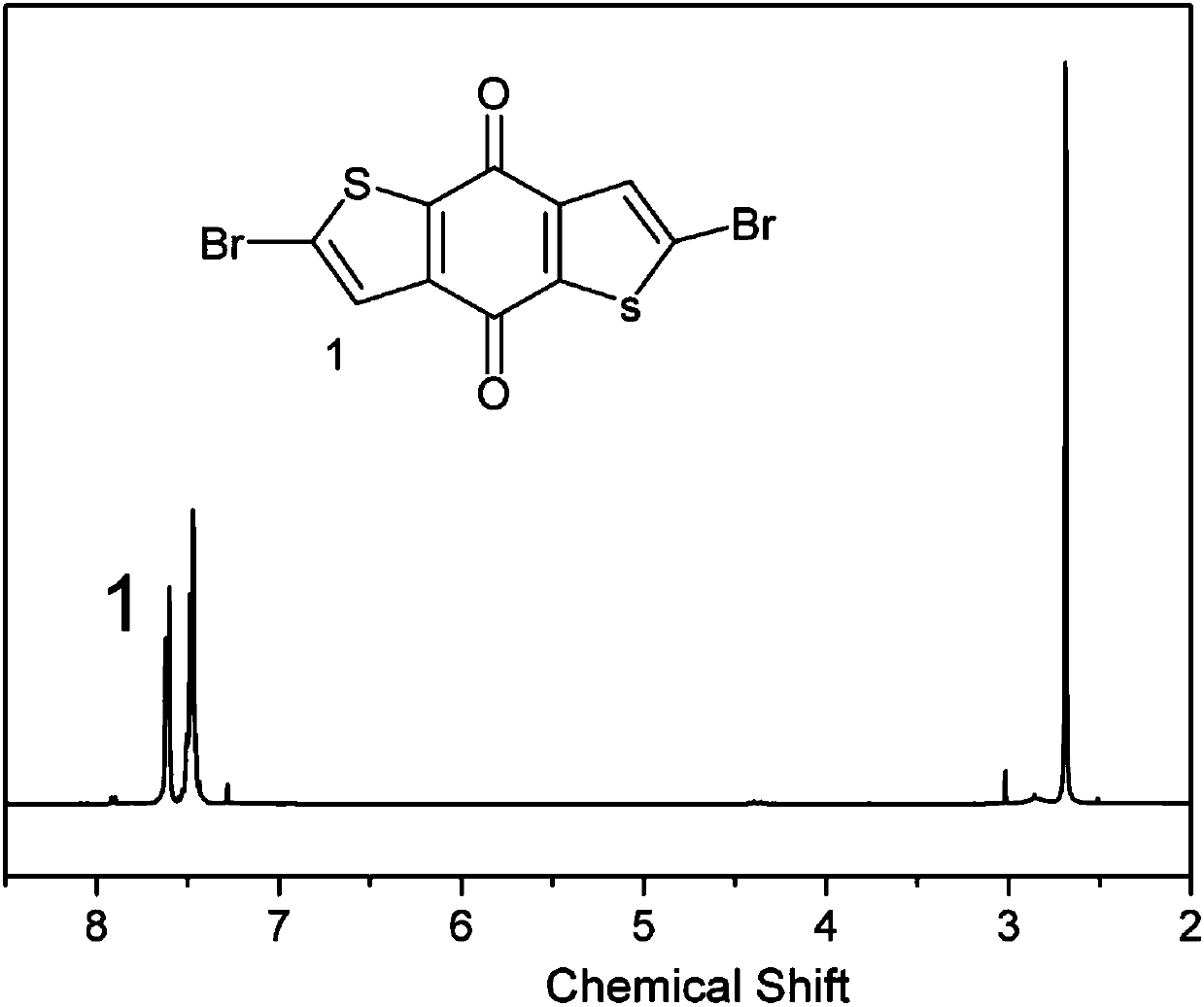
[0032] Weigh benzodithiophenedione (220mg, 1mmol) and dissolve in 30mL anhydrous CHCl 3 , quickly pipette the liquid bromine (400mg, 2.5mmol) mixed with 10mL anhydrous CHCl 3 Add to constant pressure dropping funnel, degas, N 2 Protection, slowly drop liquid bromine and anhydrous CHCl in a low-temperature reactor at -15°C 3 The mixed solution was stirred for 24 hours for 1 hour; saturated sodium thiosulfate solution was added to extract bromine, the organic layer was dried with anhydrous sodium sulfate for 2 hours, filtered, the organic layer was spin-dried, passed through the column quickly (PE:DCM=2:1), and collected Benzodithiophenedione bromide (M1) (198 mg, yield: 90%) was obtained as a bright yellow solid.
[0033] 1 H NMR (400MHz, CDCl 3 ):δ7.69(m,2H)
reference example 1
[0035] N,N-Dimethylthiophene-3-carboxamide (16.3g, 105.0mmol) and N-bromosuccinimide (NBS, 41.1g, 231.1mmol) were added to 150mL DMF, and stirred at room temperature for 2 hours in the dark. Then it was washed with water, extracted with ethyl acetate, and the organic phase was dried with anhydrous magnesium sulfate. After filtration, several layers were spin-dried and passed through the column. A yellow solid was obtained.
[0036] The yellow solid (4.9g, 15.4mmol, 40mL) was dissolved in ether, and tert-butyllithium (15.3mmol, 2.5M in hexane) was slowly added dropwise under ice-cooling conditions. Return to room temperature after the dropwise addition, then add saturated NH 4 Cl aqueous solution (30 mL), the precipitate was collected, and then passed through the column. Obtained yellow solid M1 (1.3 g, 37%)
Embodiment 2
[0038] Weigh monomer benzodithiophenedione bromide M1 (66mg, 0.17mmol) and 1,3,5-triynylbenzene (54mg, 0.36mmol) into a 25ml single-necked flask, add 12mL DMF and 36mL TEA, mix the solution to dissolve the powder, freeze the solution with liquid nitrogen and degas it, protect it with N2, thaw it with water at room temperature, repeat the above operations (freezing, degassing, thawing) three times in sequence, add Pd (pph 3 ) 4 (22mg, 6mol%), CuI (6mg, 10mol%), degassed, N 2 After protection, freezing, degassing, and thawing were repeated three times, the temperature was raised from room temperature to 100°C, benzodithiophenedione bromide (134 mg, 0.33 mmol) was added dropwise for one hour, and the reaction was stirred for 96 hours. After the device was cooled to room temperature, the turbid liquid was suction-filtered, and the filter residue was stirred in 5mol / L HCl solution for 2 hours and then suction-filtered. The filter residue was extracted with THF, chloroform, and ace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermogravimetric decomposition temperature | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com