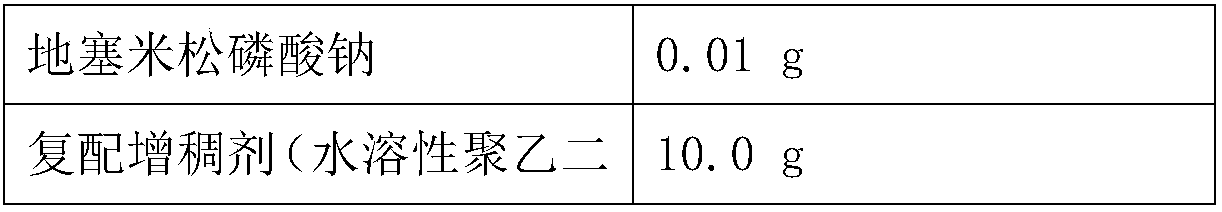Preservative-free multi-dose packaged anti-inflammatory eye drop and preparation method thereof
An eye drop and multi-dose technology, which is applied in the direction of medical preparations containing active ingredients, anti-inflammatory agents, and medical preparations with non-active ingredients, etc., can solve the problem of shortening the residence time of drugs, damaging eye health, and affecting Efficacy and other issues to achieve the effect of reducing the risk of drug contamination, avoiding bacterial invasion, and avoiding toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
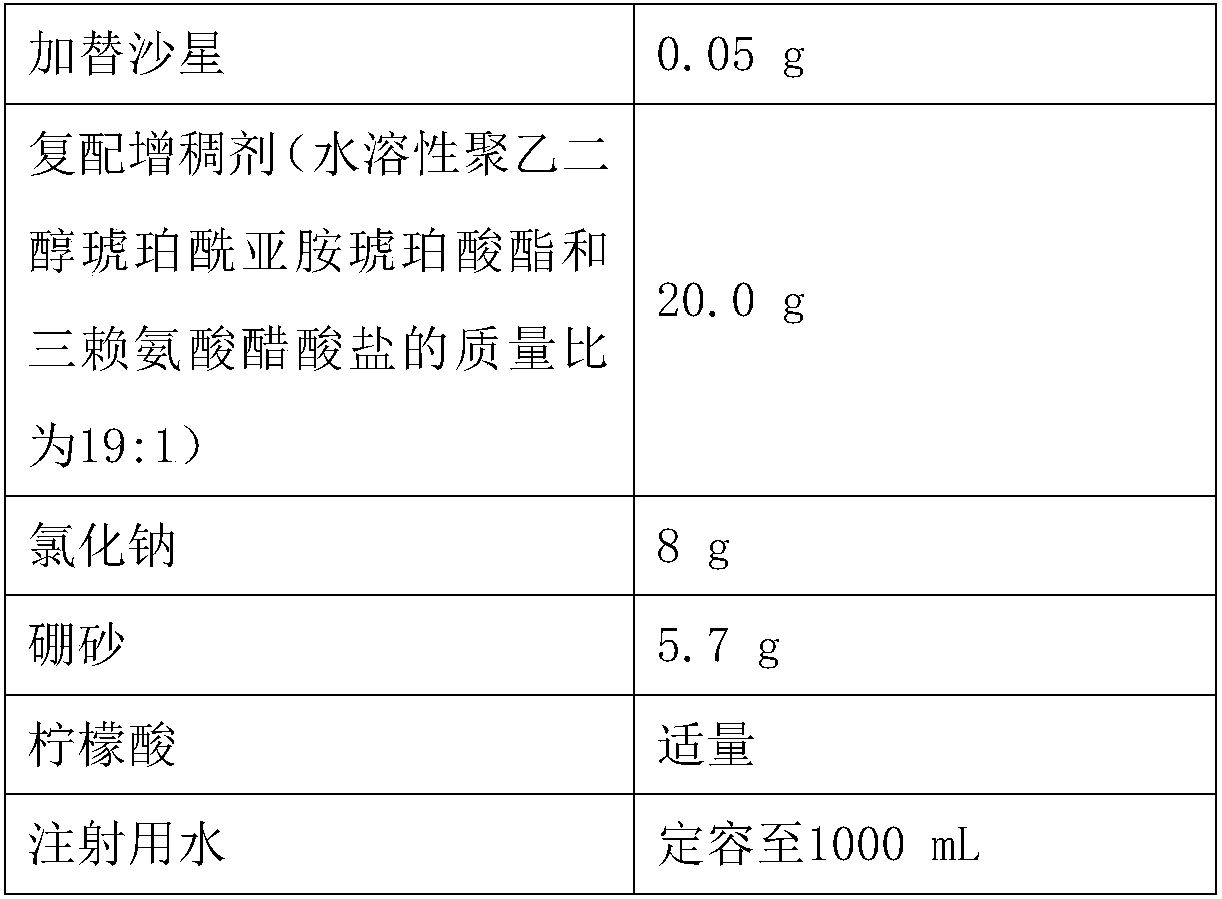
[0035] prescription
[0036]
[0037] Preparation:
[0038](1) accurately weigh gatifloxacin, sodium chloride, compound thickener in the above formula (the mass ratio of water-soluble polyethylene glycol succinimide succinate and trilysine acetate is 19:1), borax, add 300mL of water, stir to dissolve and mix evenly, adjust the pH of the solution to 6.3 with citric acid, and dilute to 1000mL with water for injection to obtain a drug solution;
[0039] (2) Filter the drug solution obtained in step 1 once through a 0.45 μm microporous membrane, and then once through a 0.22 μm microporous membrane;
[0040] (3) The liquid medicine obtained in step 2 is sterilized at 121° C. for 15 minutes by autoclaving, and then cooled;
[0041] (4) After testing the medicinal solution obtained in step 3, after passing the test, fill the medicinal solution into an eye drop bottle with a bacterial filtration device, 10 mL per bottle, and then seal it to obtain the finished product.
Embodiment 2
[0043] prescription
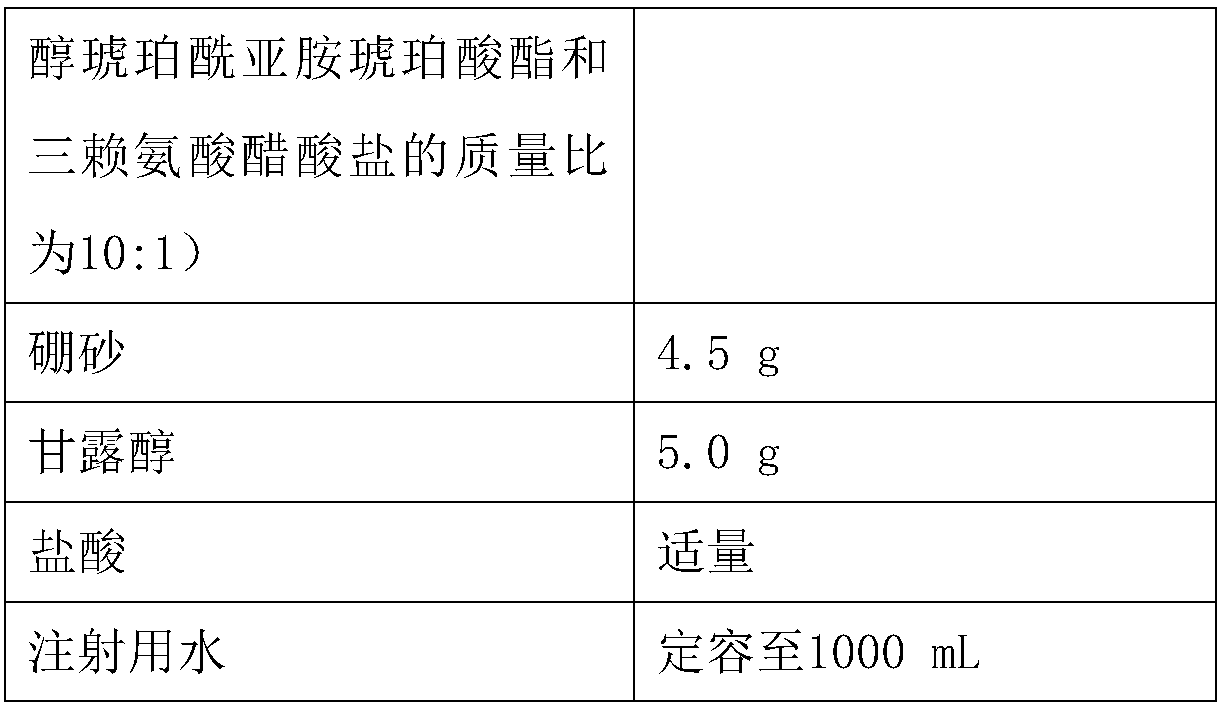
[0044]
[0045]
[0046] Preparation:
[0047] (1) Accurately weigh dexamethasone sodium phosphate, compound thickener (water-soluble polyethylene glycol succinimide succinate and trilysine acetate in a mass ratio of 10:1) in the above formula ), borax, mannitol, add 300mL of water, stir to dissolve and mix evenly, adjust the pH of the solution to 5.0 with hydrochloric acid, and set the volume to 1000mL with water for injection to obtain a drug solution;
[0048] (2) Filter the drug solution obtained in step 1 once through a 0.45 μm microporous membrane, and then once through a 0.22 μm microporous membrane;
[0049] (3) The liquid medicine obtained in step 2 is sterilized at 121° C. for 25 minutes by autoclaving, and then cooled;
[0050] (4) After testing the medicinal solution obtained in step 3, after passing the test, fill the medicinal solution into an eye drop bottle with a bacterial filtration device, 10 mL per bottle, and then seal it to o...
Embodiment 3
[0052] prescription
[0053]
[0054]
[0055] Preparation:
[0056] (1) Accurately weigh moxifloxacin hydrochloride, compound thickener (the mass ratio of water-soluble polyethylene glycol succinimide succinate and trilysine acetate is 40:1), glycerin, borax , add 300mL of water, stir to dissolve and mix evenly, adjust the pH of the solution to 9.0 with acetic acid, and then dilute to 1000mL with water for injection to obtain a drug solution;
[0057] (2) Filter the drug solution obtained in step 1 once through a 0.45 μm microporous membrane, and then once through a 0.22 μm microporous membrane;
[0058] (3) The liquid medicine obtained in step 2 is sterilized at 121° C. for 45 minutes by autoclaving, and then cooled;
[0059] (4) After testing the medicinal solution obtained in step 3, after passing the test, fill the medicinal solution into an eye drop bottle with a bacterial filtration device, 10 mL per bottle, and then seal it to obtain the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com