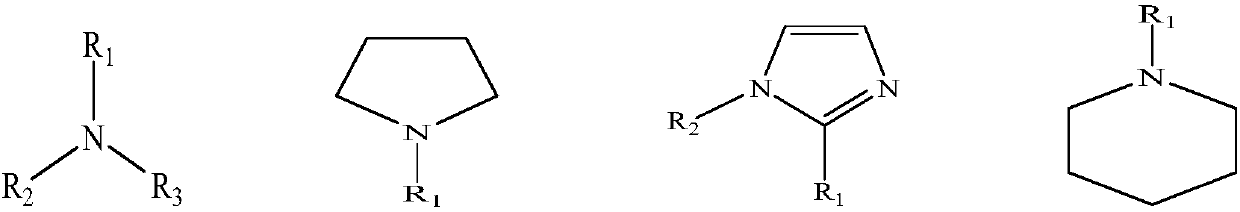
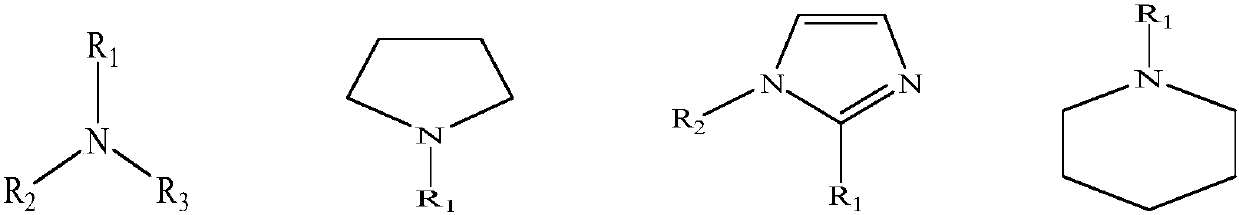
Preparation method of tetrafluoroborate quaternary ammonium salt
A technology of tetrafluoroboric acid and quaternary ammonium salt, which is applied in the field of preparation of tetrafluoroborate quaternary ammonium salt, can solve the problems of complex process conditions, low activity of alkyl carbonate, increased production cost, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add trimethyl borate (10.39 g, 0.1 mol), triethylamine (15.15 g, 0.15 mol), and 20 mL of methanol into the autoclave, stir, and react at about 135 ° C for 9 h. After the reaction was completed, the solvent was removed by rotary evaporation to obtain methyltriethylboronic acid quaternary ammonium salt.
[0021] Add 40% hydrofluoric acid aqueous solution (22.51g, 0.45mol) dropwise to the methyltriethylboronic acid quaternary ammonium salt solution, react at about 40°C for 4h, and spin off the solvent to obtain a crude product. It was recrystallized from ethanol to obtain 13.90 g of methyltriethylammonium tetrafluoroborate.
[0022] 1 H-NMR (D 2 O)δ, ppm: 1.29~1.33(t, 9H, N-CH 2 -CH 3 ), 2.93~3.01 (s, 3H, N-CH 3 ), 3..30~3.34 (q, 6H, N-CH 2 ).
[0023] 13 C-NMR (D 2 O):
[0024]
[0025] High resolution mass spectrometry: M + =116.144, M - =87.004.
[0026] Detected by ion chromatography, the results are as follows:
[0027]
Embodiment 2
[0029] Add trimethyl borate (10.39g, 0.1mol), N-methylpyrrolidine (12.77g, 0.15mol), and 20mL of methanol into the autoclave, stir, and react at about 130°C for 9h. After the reaction is completed, the solvent is removed by rotary evaporation to obtain N,N-dimethylpyrrole boric acid quaternary ammonium salt.
[0030] Add 40% hydrofluoric acid aqueous solution (22.51g, 0.45mol) dropwise to the N,N-dimethylpyrrole boric acid quaternary ammonium salt solution, react at about 40°C for 4h, and spin off the solvent to obtain a crude product. Recrystallized from ethanol to obtain 15.75 g of N,N-dimethylpyrrole ammonium tetrafluoroborate.
[0031] 1 H-NMR (D 2 O) δ, ppm: 2.21 ~ 2.35 (t, 4H, CH 2 ), 3.08~3.15 (s, 6H, N-CH 3 ), 3.56~3.64 (q, 4H, N-CH 2 ).
[0032] 13 C-NMR (D 2 O):
[0033]
[0034] High resolution mass spectrometry: M + =100.113, M - =87.004.
[0035] Detected by ion chromatography, the results are as follows:
[0036]
Embodiment 3
[0038] Add trimethyl borate (10.39g, 0.1mol), 1-methylimidazole (12.32g, 0.15mol), and 20mL of methanol into the autoclave, stir, and react at about 125°C for 7h. After the reaction is completed, the solvent is removed by rotary evaporation to obtain 1,3-dimethylimidazole boronic acid quaternary ammonium salt.
[0039] Add 40% hydrofluoric acid aqueous solution (22.51g, 0.45mol) dropwise to 1,3-dimethylimidazole boric acid quaternary ammonium salt solution, react at about 40°C for 4h, and spin evaporate to remove the solvent to obtain a crude product. Recrystallized from ethanol to obtain 14.36 g of 1,3-dimethylimidazolium ammonium tetrafluoroborate.
[0040] 1 H-NMR (D 2 O)δ, ppm: 3.79~3.86(s, 6H, CH 3 ), 7.43-7.52 (s, 2H, CH), 8.70-8.83 (s, 1H, CH).
[0041] 13 C-NMR (D 2 O):
[0042]
[0043] High resolution mass spectrometry: M + =97.135, M - =87.004. Detected by ion chromatography, the results are as follows:
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com