Hemostasis fibrous membrane, preparation method of hemostasis fibrous membrane and hemostasis product adopting hemostasis fibrous membrane
A technology of hemostatic fibers and microfibers, which is used in medical science, absorbent pads, bandages, etc., can solve the problems of unsatisfactory hemostatic effect of film-like hemostatic materials, inability to achieve fast and efficient hemostasis, poor adherence and adhesion, etc. Excellent tissue adhesion, significant hemostatic effect, high water absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
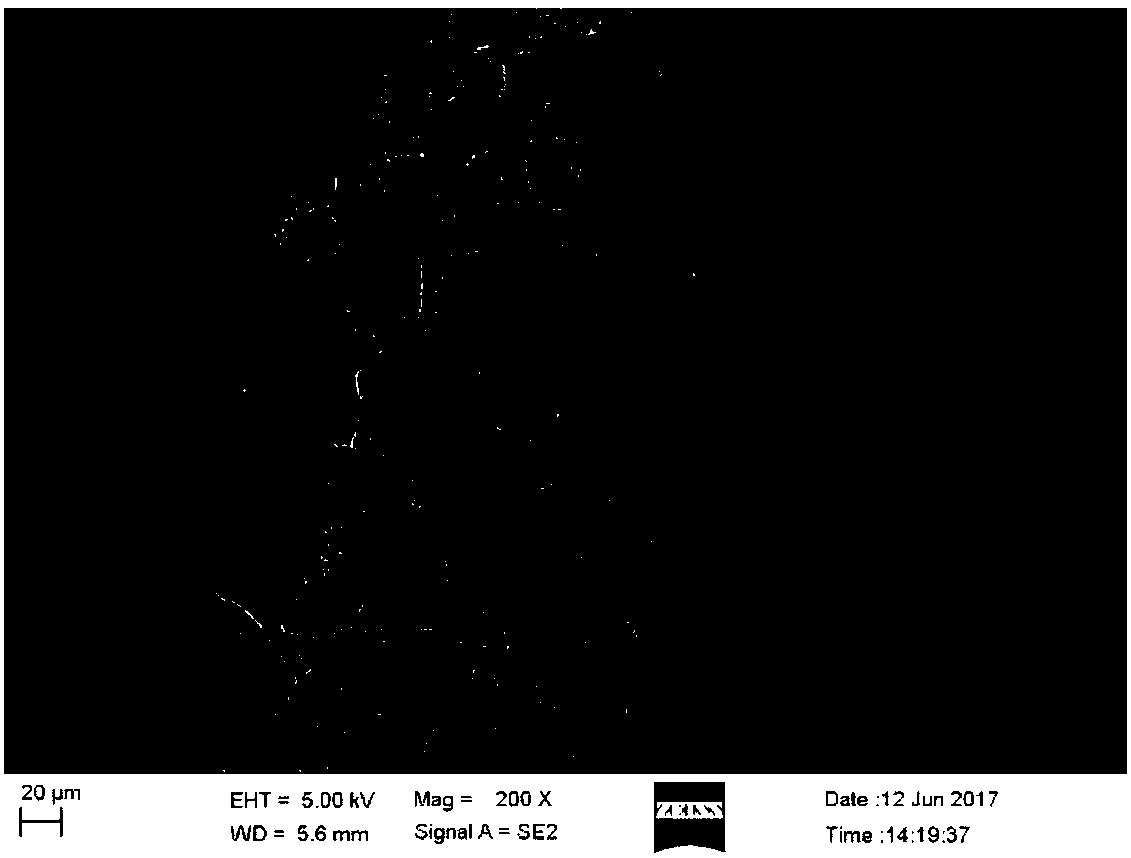
[0055] The first embodiment of the present invention provides a hemostatic fibrous membrane. The hemostatic fiber membrane includes microfibers, and the microfibers have an interlaced structure formed by overlapping short nanofibers. The microfibers are derived from crosslinked nanofibrous material.
[0056] Wherein, the crosslinked nanofiber material can be obtained by performing crosslinking modification on the nanofiber material.
[0057]
[0058] The nanofibrous material of the present invention can be derived from a biocompatible and biodegradable polymer material, more preferably a hydrophilic polymer material. For example, the polymer material may include one or a combination of two or more of collagen (Collagen), chitosan (Chitosan), hyaluronic acid (HA), alginate, cellulose and derivatives thereof.
[0059] The nanofibrous material of the present invention can be formed by interweaving fiber filaments. Preference is given to nanofibrous materials produced using e...
no. 2 approach
[0078] The second embodiment of the present invention provides a method for preparing a hemostatic fibrous membrane, comprising the following steps:
[0079] Electrospinning step: preparing the nanofiber material by electrospinning;
[0080] Crosslinking step: performing crosslinking treatment on the nanofiber material in the presence of a crosslinking agent to obtain a crosslinked nanofiber material;
[0081] Shearing step: performing shearing treatment on the cross-linked nanofiber material to obtain microfibers;
[0082] Forming step: using a mold to press the microfibers to obtain a molded body.
[0083] The "cross-linking" and "cross-linking modification" described herein have the same or similar meanings, and in the process of "cross-linking", some characteristics of "modification" can be attached. For the sake of simplicity, in the present invention, Use "cross-linked" instead of "cross-linked modification".
[0084]
[0085] In the electrospinning step, the fiber ...
no. 3 approach
[0114] The third embodiment of the present invention also provides a hemostatic product, including the hemostatic fibrous membrane according to the first embodiment of the present invention, or the hemostatic fibrous membrane prepared according to the method for preparing the hemostatic fibrous membrane according to the second embodiment of the present invention.
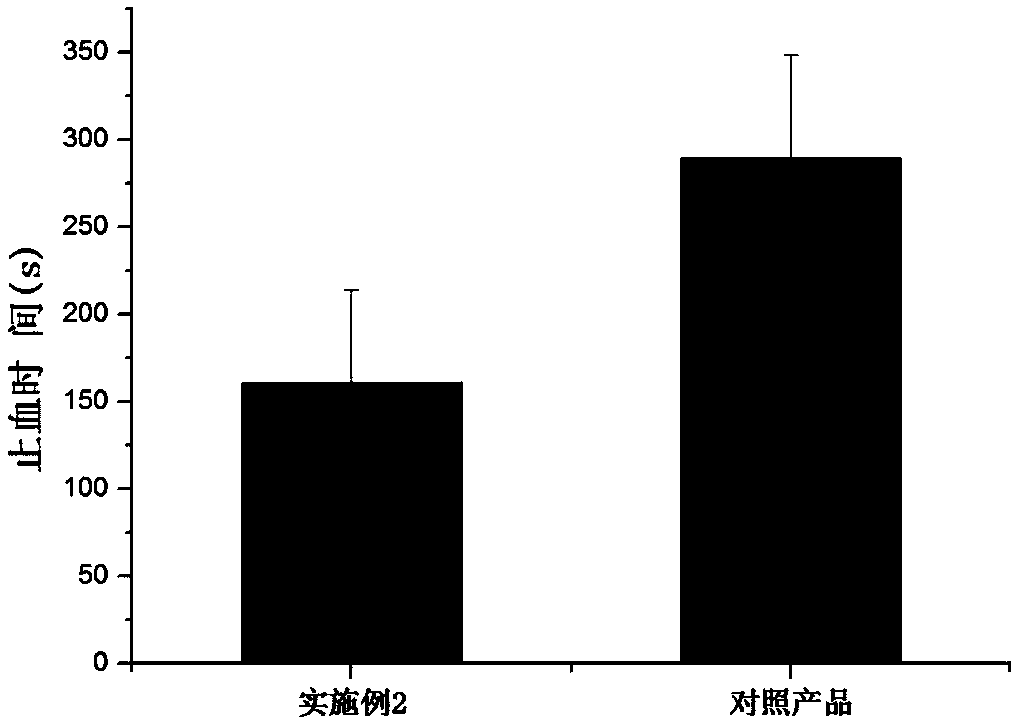
[0115] The hemostatic product of the present invention can be used for hemostasis and repair when tissue oozes, capillary hemorrhage, arteriole hemorrhage, and lacunar ooze, and / or, for hemostasis and repair of burns, wounds, and surgical wounds. application prospects. When it is used for hemostasis and repair when the cavity oozes, it can be applied to the oozing of the cavity and other parts with the help of auxiliary equipment, or the doctor can use this product in combination with other commercially available products based on experience, such as Hemostatic sponge, hemostatic gauze and other products to achieve ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com