Polyquinoline polymer material and preparation method thereof
A technology of polymer materials and polyquinoline, which is applied in the direction of luminescent materials, chemical instruments and methods, etc., can solve the problems of low yield of polymers, and achieve the effects of good solubility, easy synthesis, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
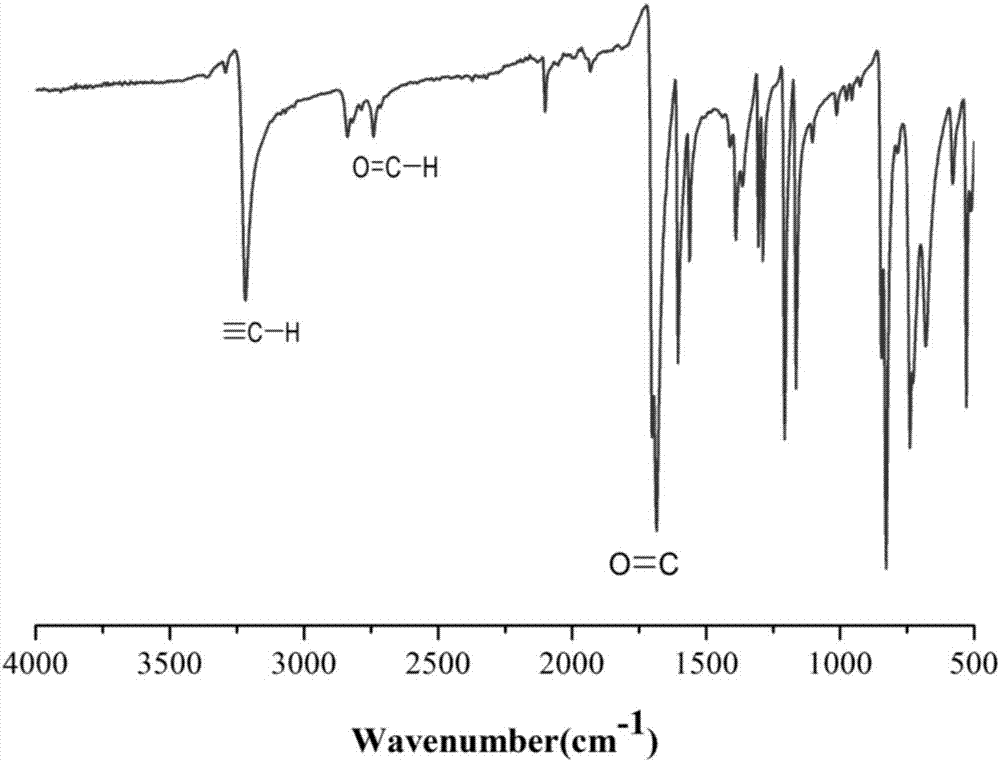
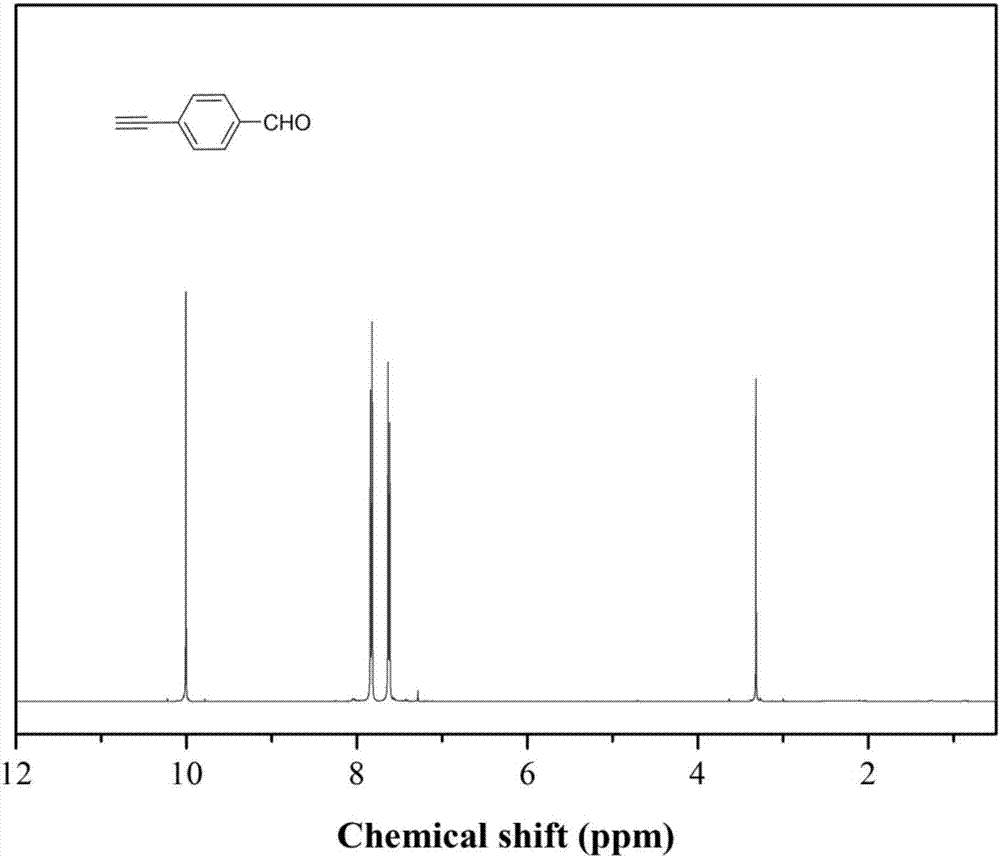
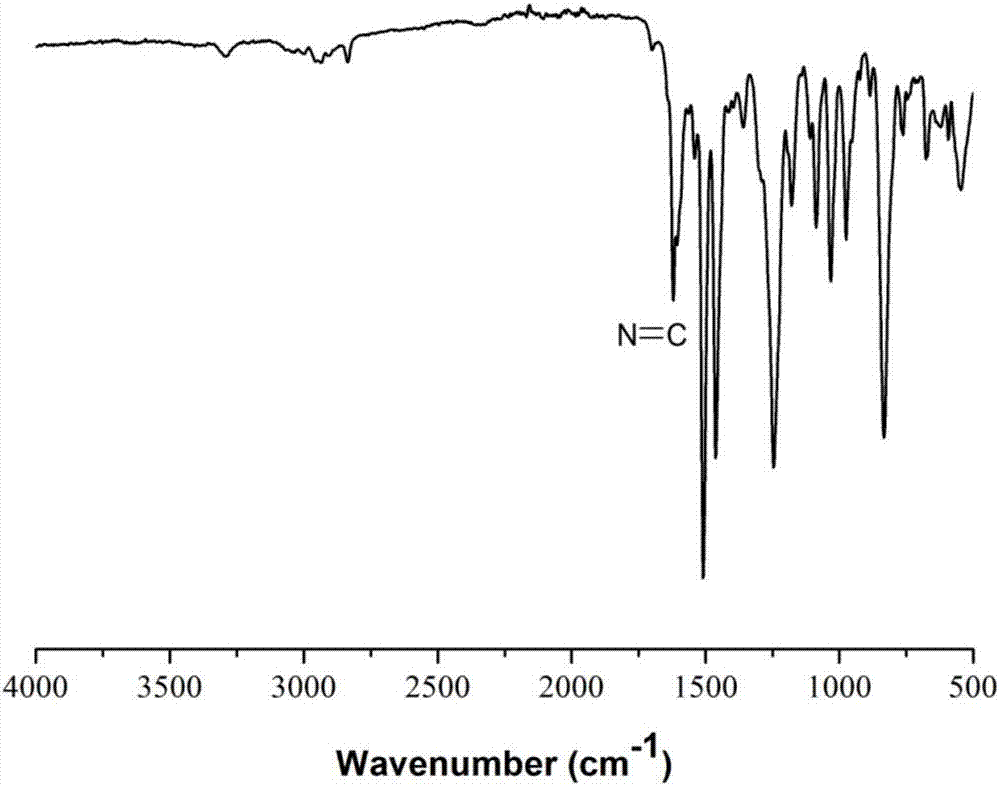
[0040] Add 7.4g (40mmol) p-bromobenzaldehyde, 701mg (1mmol) bistriphenylphosphine palladium dichloride, 190mg (1mmol) cuprous iodide, 524g (2mmol) triphenylphosphine in the reaction bottle of 500mL, extract Nitrogen was filled after vacuum to remove oxygen in the system, 150mL triethylamine was added, the temperature was raised to 65°C, and after stirring for 30min, 8.48mL (60mmol) trimethylsilylacetylene was added, reacted for 24 hours, and triethylamine was removed by rotary evaporation. The intermediate product 1 was obtained; the intermediate product 1 was dissolved in dichloromethane, washed with water, and the organic phase was separated by column chromatography using petroleum ether / dichloromethane as the eluent to obtain the intermediate product 2.
[0041] Add 2.02g (10mmol) of intermediate product 2, 1g (10mmol) of sodium carbonate, and 100mL of methanol into a 500mL reaction flask. After stirring at room temperature for 24 hours, the methanol was removed by rotary e...
Embodiment 2
[0044] In a 500mL reaction flask, add 7.64g (40mmol) 5-bromothiophene-2-carbaldehyde, 701mg (1mmol) bistriphenylphosphine palladium dichloride, 190mg (1mmol) cuprous iodide, 524g (2mmol) triphenyl In order to remove the oxygen in the system, add 150mL of triethylamine, raise the temperature to 45°C, stir for 30min, add 8.48mL (60mmol) trimethylsilylacetylene, react for 24 hours, and remove the three ethylamine to obtain intermediate product 1; intermediate product 1 was dissolved in dichloromethane, washed with water, and the organic phase was separated by column chromatography using petroleum ether / dichloromethane as eluent to obtain intermediate product 2.
[0045] Add 2.16g (10mmol) of intermediate product 2, 1g (10mmol) of sodium carbonate, and 100mL of methanol into a 500mL reaction flask. Stir at room temperature for 24 hours, remove methanol by rotary evaporation, and obtain intermediate product 3; add dichloromethane to dissolve intermediate product 3, wash with water, a...
Embodiment 3
[0048] In a 500mL reaction flask, add 11.4g (40mmol) 9-bromoanthracene-10-formaldehyde, 701mg (1mmol) bistriphenylphosphine palladium dichloride, 190mg (1mmol) cuprous iodide, 524g (2mmol) triphenyl In order to remove the oxygen in the system, add 150mL of triethylamine, raise the temperature to 65°C, stir for 30min, add 8.48mL (60mmol) of trimethylsilylacetylene, react for 24 hours, and remove the trimethylsilylacetylene by rotary evaporation. ethylamine to obtain intermediate product 1; intermediate product 1 was dissolved in dichloromethane, washed with water, and the organic phase was separated by column chromatography using petroleum ether / dichloromethane as eluent to obtain intermediate product 2.
[0049] Add 3.02g (10mmol) of intermediate product 2, 1g (10mmol) of sodium carbonate, and 100mL of methanol into a 500mL reaction flask. Stir at room temperature for 24 hours, remove methanol by rotary evaporation, and obtain intermediate product 3; add dichloromethane to dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com