Garnet structure solid electrolyte material and preparation method thereof
A solid electrolyte and garnet technology, applied in the manufacture of electrolyte batteries, electrolytes, non-aqueous electrolyte batteries, etc., can solve the problems of reducing ceramic density and ionic conductivity, affecting the electrochemical performance of solid electrolytes, and reducing lithium ion content too much , to achieve good chemical stability, chemical stability, simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
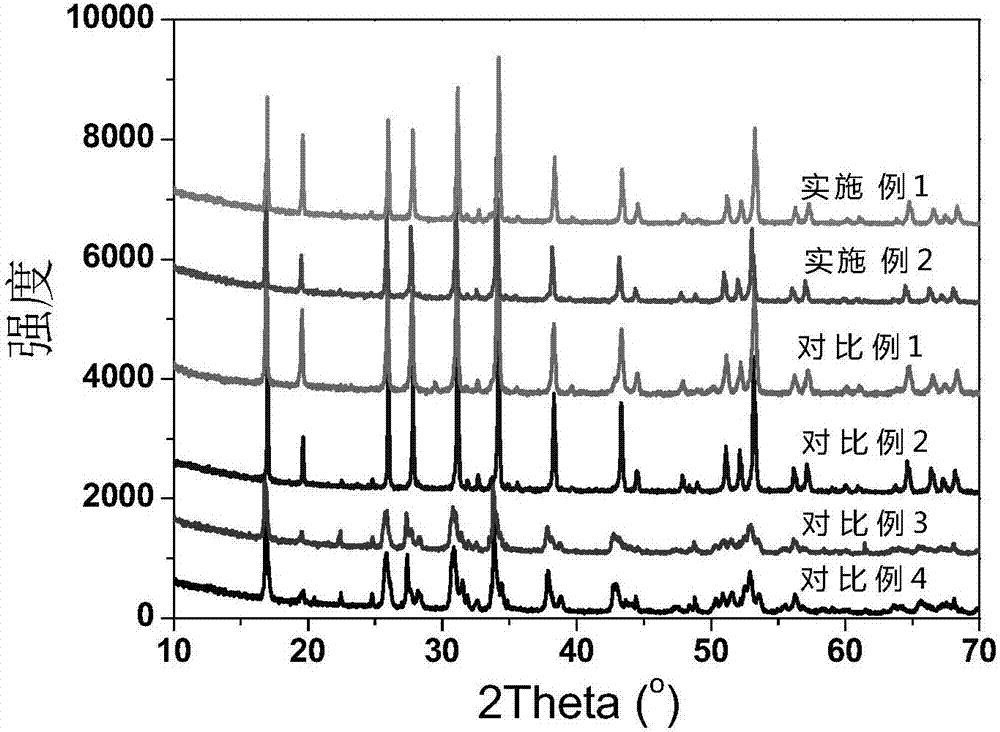
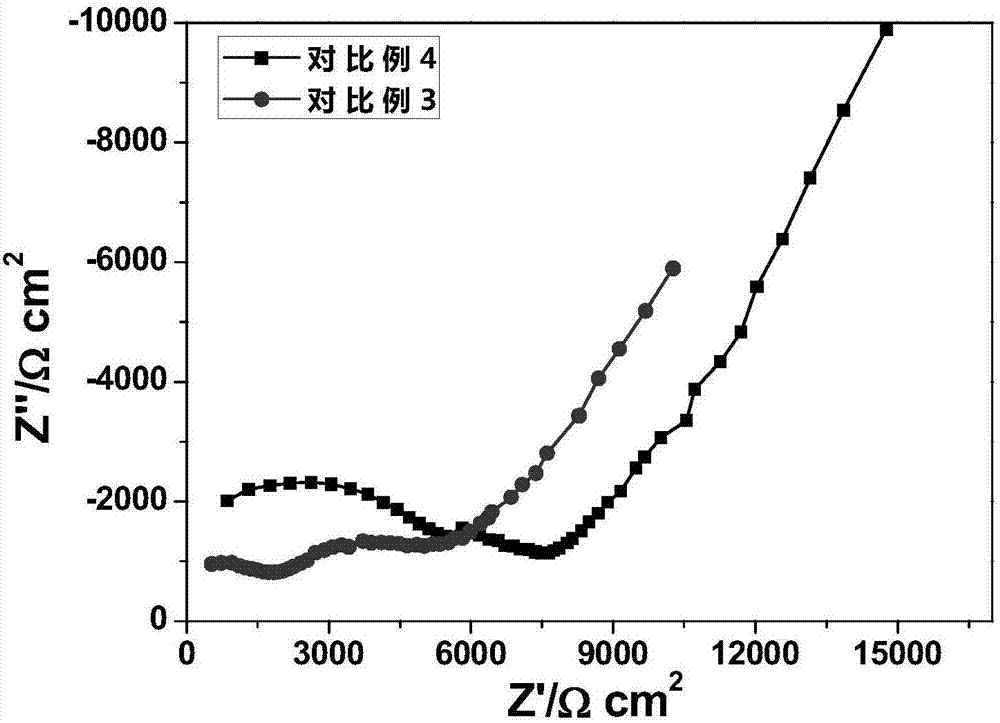
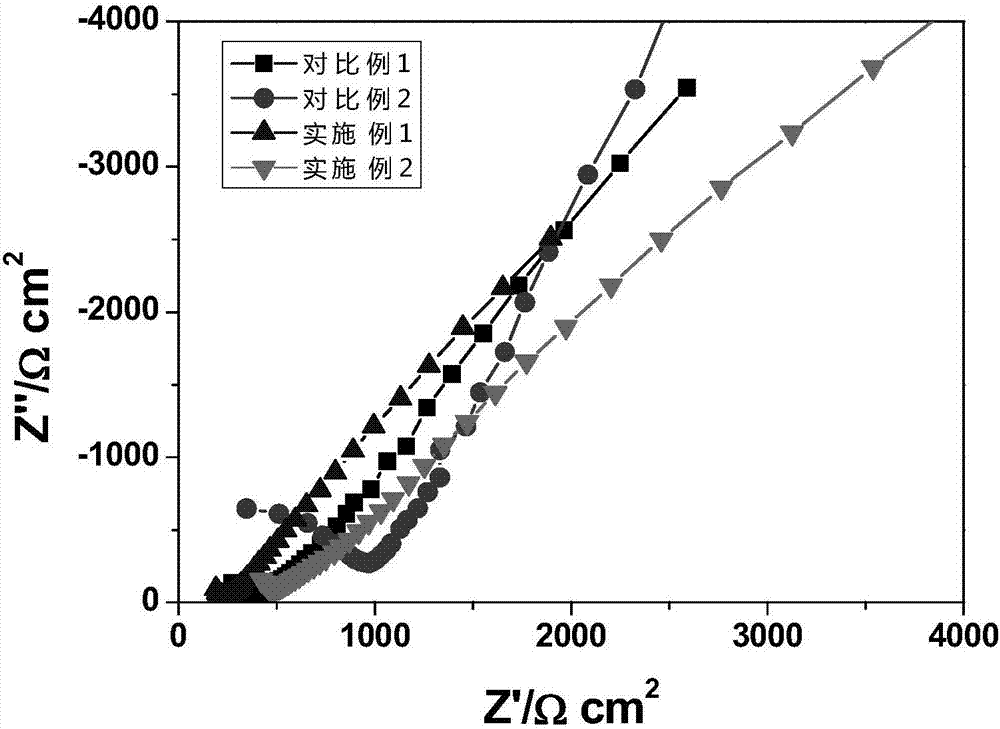
Image
Examples
Embodiment 1
[0032] The preparation chemical composition is Li 6.05 Ca 0.05 La 2.95 Ta 1.0 Zr 1.0 o 12 Lithium carbonate as a lithium source, lanthanum oxide as a lanthanum source, zirconium acetate as a zirconium source, calcium carbonate as a calcium source, tantalum pentoxide as a source, and acetic acid as a mixed medium. According to the chemical composition Li 6.05 Ca 0.05 La 2.95 Ta 1.0 Zr 1.0 o 12 Proportionally weigh lithium carbonate, lanthanum oxide, zirconium acetate, calcium carbonate, and tantalum pentoxide and add them to acetic acid. The excess of lithium carbonate is 30% to compensate for the volatilization loss of lithium during the preparation process. Stir magnetically at 50°C until The solvent is all volatilized to obtain the precursor mixed powder. The precursor powder was heated to 400°C at a heating rate of 10°C / min and then kept for 2 hours, then heated to 750°C at a heating rate of 10°C / min and then calcined for 8 hours, then cooled to room temperature ...
Embodiment 2
[0034] The preparation chemical composition is Li 6.45 Ca 0.05 La 2.95 Ta 0.6 Zr 1.4 o 12 Lithium carbonate as a lithium source, lanthanum oxide as a lanthanum source, zirconium acetate as a zirconium source, calcium carbonate as a calcium source, tantalum pentoxide as a source, and acetic acid as a mixed medium. According to the chemical composition Li 6.45 Ca 0.05 La 2.95 Ta 0.6 Zr 1.4 o 12 Proportionally weigh lithium carbonate, lanthanum oxide, zirconium acetate, calcium carbonate, and tantalum pentoxide and add them to acetic acid. The excess of lithium carbonate is 30% to compensate for the volatilization loss of lithium during the preparation process. Stir magnetically at 50°C until The solvent is all volatilized to obtain the precursor mixed powder. The precursor powder was heated to 400°C at a heating rate of 10°C / min and then kept for 2 hours, then heated to 750°C at a heating rate of 10°C / min and then calcined for 8 hours, then cooled to room temperature ...
Embodiment 3
[0047] The preparation chemical composition is Li 6.05 Ca 0.05 La 2.95 Ta 0.05 Zr 1.95 o 12 The solid electrolyte material, and using the same raw material as in Example 1, was magnetically stirred at 100° C. until the solvent was completely volatilized, and the precursor mixed powder was obtained. The precursor powder was heated to 300°C at a heating rate of 1°C / min and then kept for 8 hours, then heated to 600°C at a heating rate of 1°C / min and then calcined for 24 hours, then cooled to room temperature at a cooling rate of 1°C / min. get Li 6.05 Ca 0.05 La 2.95 Ta 0.05 Zr 1.95 o 12 Electrolyte powder, the Li 6.05 Ca 0.05 La 2.95 Ta 0.05 Zr 1.95 o 12 The powder was kept under 10MPa pressure for 2h, pressed into a disc with a diameter of 14mm and a thickness of 1mm, and the disc was placed in Li 6.05 Ca 0.05 La 2.95 Ta 0.05 Zr 1.95 o 12 Covered with electrolyte powder, the temperature was raised to 1000°C at a heating rate of 1°C / min and sintered for 24 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com