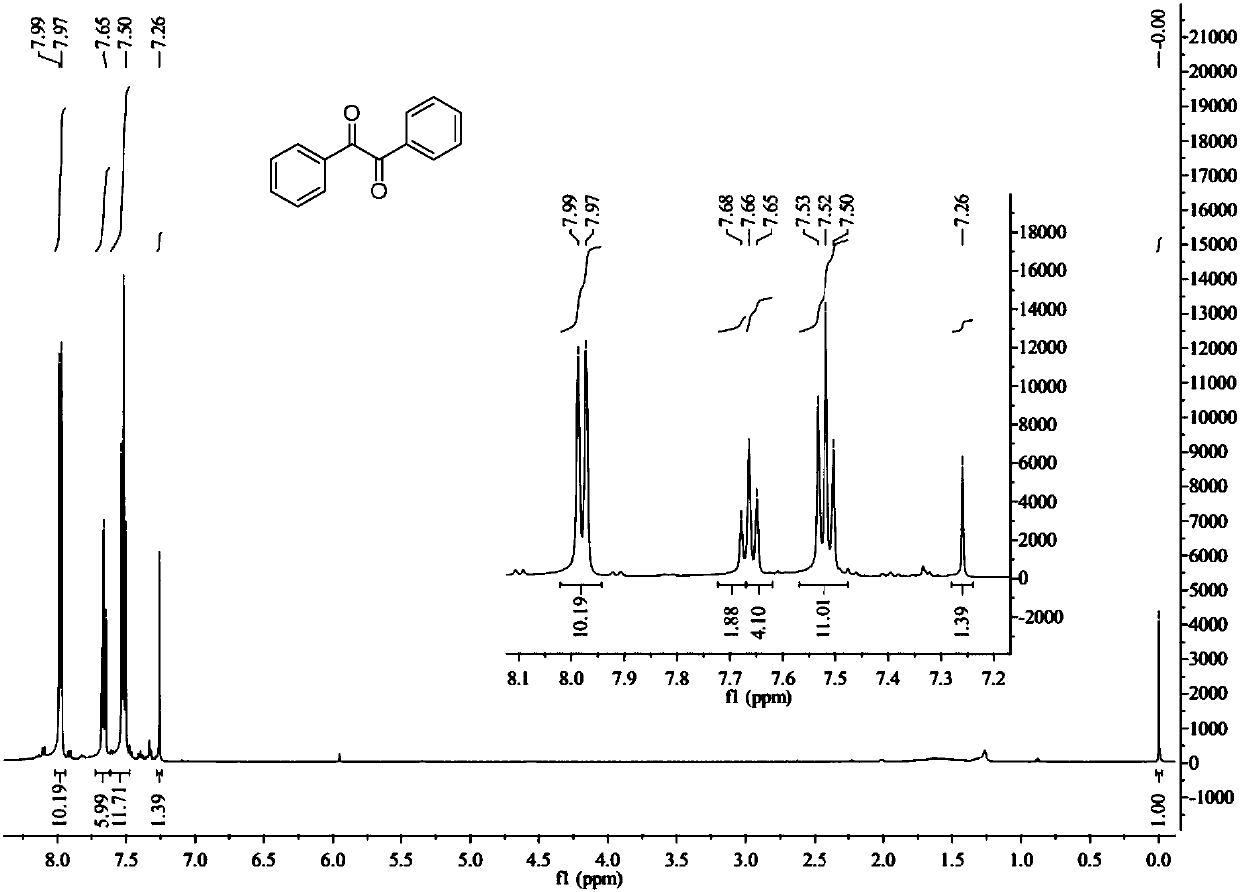
Method for preparing benzil by performing catalytic oxidation on benzoin
A technology for catalytic oxidation and benzoin, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of complicated preparation of complexes, high yield of benzil, and short reaction time, and achieves economical The effect of removing catalyst preparation process and reaction process is green and environmentally friendly, and the reaction conditions are mild
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
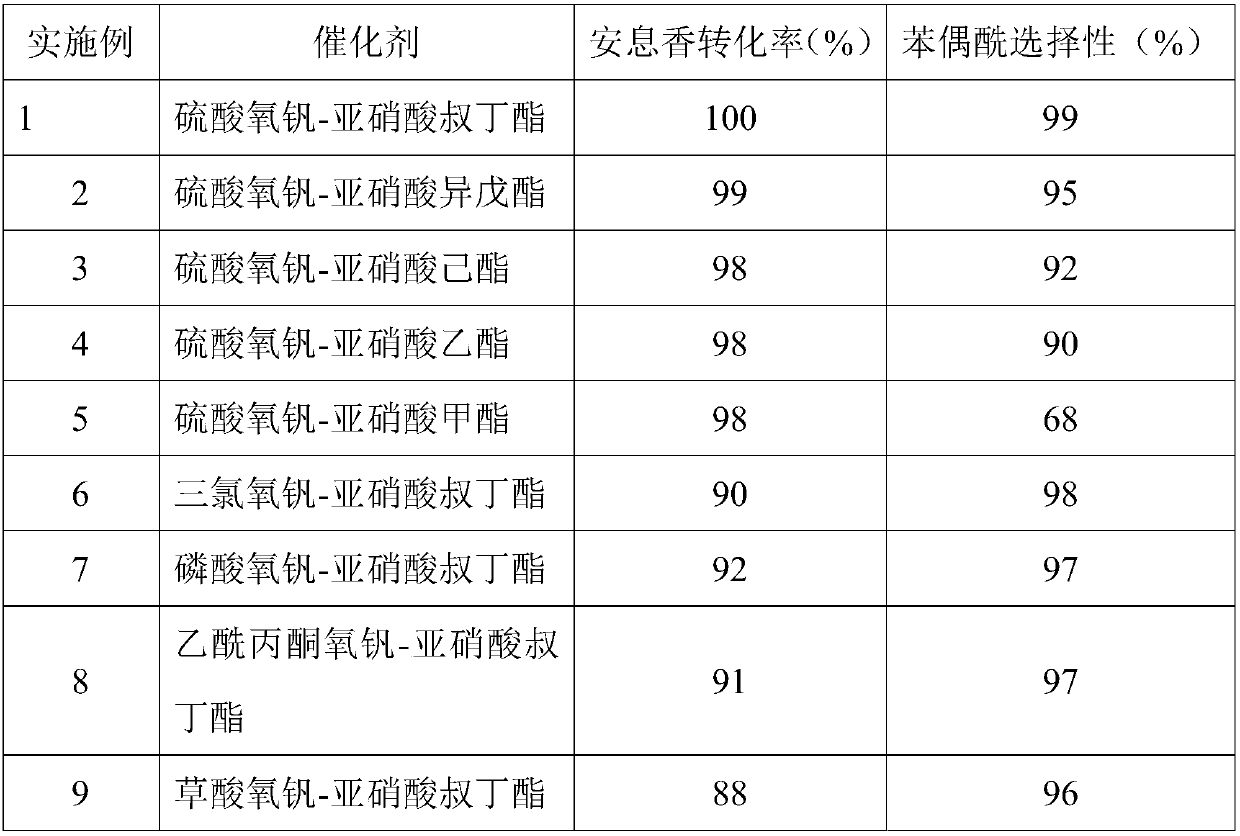
[0024] Embodiment 1-9: The impact of different catalyst types on the conversion rate of benzoin and the selectivity of benzil, the specific experimental process is described as follows:
[0025] 5mmol benzoin, 2mol% vanadyl oxy compound, 2mol% nitrite, 10ml acetonitrile are added in the 25ml reactor, after the reactor is sealed, fill the reactor with 0.5MPaO 2 , the temperature was raised to 100° C. under stirring, and kept for 6 hours. After the reaction was completed, it was cooled to room temperature, and the product was analyzed quantitatively by gas chromatography.
[0026] Table 1: The effect of different catalyst types on the conversion rate of benzoin and the selectivity of benzil
[0027]
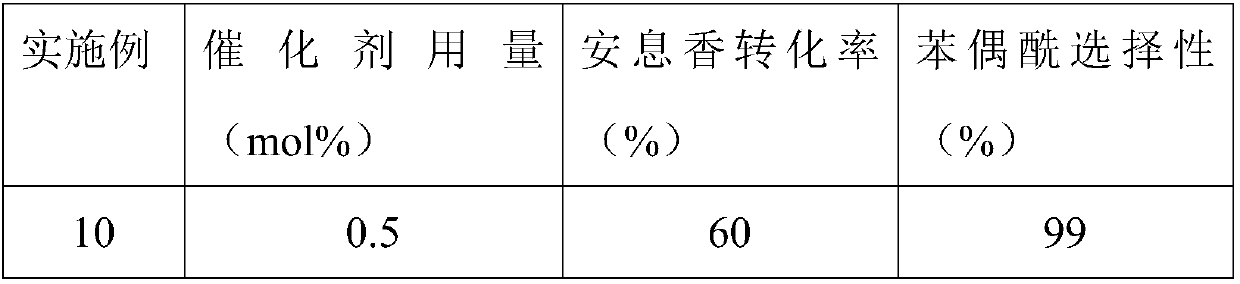
Embodiment 10-15
[0028] Embodiment 10-15: The addition amount of catalyst affects the conversion rate of benzoin and the selectivity of benzil, and the specific experimental process is described as follows:
[0029] A certain amount of catalyst (vanadyl sulfate / tert-butyl nitrite=100mol%), 5mmol benzoin, and 10ml acetonitrile are added in a 25ml reactor, and 0.5MPa O is charged in the reactor. 2 , the temperature was raised to 100° C. under stirring, and kept for 6 hours. After the reaction was completed, it was cooled to room temperature, and the product was analyzed quantitatively by gas chromatography.
[0030] Table 2: The effect of the amount of catalyst added on the conversion rate of benzoin and the selectivity of benzil
[0031]
[0032]
Embodiment 16-19
[0033] Embodiment 16-19: the influence of the molar ratio of nitrite and vanadyl compound on the conversion rate of benzoin and the selectivity of benzil, the specific experimental process is described as follows:
[0034] 5mmol benzoin, 2mol% vanadyl sulfate, a certain amount of nitrite, 10ml acetonitrile add in the 25ml reactor, fill in 0.5MPa O in the reactor 2 , the temperature was raised to 100° C. under stirring, and kept for 6 hours. After the reaction was completed, it was cooled to room temperature, and the product was analyzed quantitatively by gas chromatography.
[0035] Table 3: The effect of the molar ratio of nitrite to vanadyl on the conversion of benzoin and the selectivity of benzil
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com